Role of Toll-Like Receptor Signaling in the Pathogenesis of Graft-versus-Host Diseases
- PMID: 27529218
- PMCID: PMC5000685
- DOI: 10.3390/ijms17081288
Role of Toll-Like Receptor Signaling in the Pathogenesis of Graft-versus-Host Diseases
Abstract
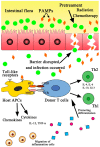
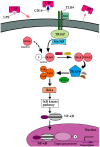
Graft-versus-host disease (GVHD) and infection are major complications after allogeneic hematopoietic stem cell transplantation (allo-HSCT) and the leading causes of morbidity and mortality in HSCT patients. Recent work has demonstrated that the two complications are interdependent. GVHD occurs when allo-reactive donor T lymphocytes are activated by major histocompatibility antigens or minor histocompatibility antigens on host antigen-presenting cells (APCs), with the eventual attack of recipient tissues or organs. Activation of APCs is important for the priming of GVHD and is mediated by innate immune signaling pathways. Current evidence indicates that intestinal microbes and innate pattern-recognition receptors (PRRs) on host APCs, including both Toll-like receptors (TLRs) and nucleotide oligomerization domain (NOD)-like receptors (NLRs), are involved in the pathogenesis of GVHD. Patients undergoing chemotherapy and/or total body irradiation before allo-HSCT are susceptible to aggravated gastrointestinal epithelial cell damage and the subsequent translocation of bacterial components, followed by the release of endogenous dangerous molecules, termed pathogen-associated molecular patterns (PAMPs), which then activate the PRRs on host APCs to trigger local or systemic inflammatory responses that modulate T cell allo-reactivity against host tissues, which is equivalent to GVHD. In other words, infection can, to some extent, accelerate the progression of GVHD. Therefore, the intestinal flora's PAMPs can interact with TLRs to activate and mature APCs, subsequently activate donor T cells with the release of pro-inflammatory cytokines, and eventually, induce GVHD. In the present article, we summarize the current perspectives on the understanding of different TLR signaling pathways and their involvement in the occurrence of GVHD.
Keywords: GVHD; HSCT; PAMPs; TLRs.
Figures
Similar articles
-
Toll-Like Receptor Stimulation by MicroRNAs in Acute Graft-vs.-Host Disease.Front Immunol. 2018 Nov 5;9:2561. doi: 10.3389/fimmu.2018.02561. eCollection 2018. Front Immunol. 2018. PMID: 30455702 Free PMC article. Review.
-
STING negatively regulates allogeneic T-cell responses by constraining antigen-presenting cell function.Cell Mol Immunol. 2021 Mar;18(3):632-643. doi: 10.1038/s41423-020-00611-6. Epub 2021 Jan 26. Cell Mol Immunol. 2021. PMID: 33500563 Free PMC article.
-
The role of pattern-recognition receptors in graft-versus-host disease and graft-versus-leukemia after allogeneic stem cell transplantation.Front Immunol. 2014 Jul 18;5:337. doi: 10.3389/fimmu.2014.00337. eCollection 2014. Front Immunol. 2014. PMID: 25101080 Free PMC article. Review.
-
Innate immunity and transplantation tolerance: the potential role of TLRs/NLRs in GVHD.Korean J Hematol. 2011 Jun;46(2):69-79. doi: 10.5045/kjh.2011.46.2.69. Epub 2011 Jun 21. Korean J Hematol. 2011. PMID: 21747878 Free PMC article.
-
Extracellular release of damaged mitochondria induced by prehematopoietic stem cell transplant conditioning exacerbates GVHD.Blood Adv. 2024 Jul 23;8(14):3691-3704. doi: 10.1182/bloodadvances.2023012328. Blood Adv. 2024. PMID: 38701354 Free PMC article.
Cited by
-
Syk Plays a Critical Role in the Expression and Activation of IRAK1 in LPS-Treated Macrophages.Mediators Inflamm. 2017;2017:1506248. doi: 10.1155/2017/1506248. Epub 2017 Jun 7. Mediators Inflamm. 2017. PMID: 28680194 Free PMC article.
-
Different engagement of TLR2 and TLR4 in Porphyromonas gingivalis vs. ligature-induced periodontal bone loss.Braz Oral Res. 2017 Aug 21;31:e63. doi: 10.1590/1807-3107BOR-2017.vol31.0063. Braz Oral Res. 2017. PMID: 28832712 Free PMC article.
-
Roles of the intestinal microbiota and microbial metabolites in acute GVHD.Exp Hematol Oncol. 2021 Oct 27;10(1):49. doi: 10.1186/s40164-021-00240-3. Exp Hematol Oncol. 2021. PMID: 34706782 Free PMC article. Review.
-
Global Down-regulation of Gene Expression Induced by Mouse Mammary Tumor Virus (MMTV) in Normal Mammary Epithelial Cells.Viruses. 2023 May 2;15(5):1110. doi: 10.3390/v15051110. Viruses. 2023. PMID: 37243196 Free PMC article.
-
Toll-Like Receptor Stimulation by MicroRNAs in Acute Graft-vs.-Host Disease.Front Immunol. 2018 Nov 5;9:2561. doi: 10.3389/fimmu.2018.02561. eCollection 2018. Front Immunol. 2018. PMID: 30455702 Free PMC article. Review.
References
-
- Mo X.D., Huang X.J. Life quality related to health related after allogenic transplantation. Chin. J. Hematol. 2012;33:968–971. - PubMed
-
- Van Bekkum D.W., Knaan S. Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J. Natl. Cancer Inst. 1977;58:787–790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

