Deep Artificial Neural Networks and Neuromorphic Chips for Big Data Analysis: Pharmaceutical and Bioinformatics Applications
- PMID: 27529225
- PMCID: PMC5000710
- DOI: 10.3390/ijms17081313
Deep Artificial Neural Networks and Neuromorphic Chips for Big Data Analysis: Pharmaceutical and Bioinformatics Applications
Abstract
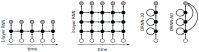
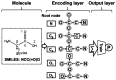
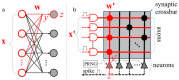
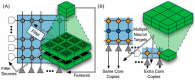
Over the past decade, Deep Artificial Neural Networks (DNNs) have become the state-of-the-art algorithms in Machine Learning (ML), speech recognition, computer vision, natural language processing and many other tasks. This was made possible by the advancement in Big Data, Deep Learning (DL) and drastically increased chip processing abilities, especially general-purpose graphical processing units (GPGPUs). All this has created a growing interest in making the most of the potential offered by DNNs in almost every field. An overview of the main architectures of DNNs, and their usefulness in Pharmacology and Bioinformatics are presented in this work. The featured applications are: drug design, virtual screening (VS), Quantitative Structure-Activity Relationship (QSAR) research, protein structure prediction and genomics (and other omics) data mining. The future need of neuromorphic hardware for DNNs is also discussed, and the two most advanced chips are reviewed: IBM TrueNorth and SpiNNaker. In addition, this review points out the importance of considering not only neurons, as DNNs and neuromorphic chips should also include glial cells, given the proven importance of astrocytes, a type of glial cell which contributes to information processing in the brain. The Deep Artificial Neuron-Astrocyte Networks (DANAN) could overcome the difficulties in architecture design, learning process and scalability of the current ML methods.
Keywords: Quantitative Structure–Activity Relationship; artificial neural networks; artificial neuron–astrocyte networks; big data; deep learning; drug design; genomic medicine; neuromorphic chips; protein structure prediction; tripartite synapses.
Figures




References
-
- Puri M., Pathak Y., Sutariya V.K., Tipparaju S., Moreno W. Artificial Neural Network for Drug Design, Delivery and Disposition. Elsevier Science; Amsterdam, The Netherlands: 2015.
-
- Yee L.C., Wei Y.C. Statistical Modelling of Molecular Descriptors in QSAR/QSPR. Volume 10. John Wiley & Sons; Hoboken, NJ, USA: 2012. Current modeling methods used in QSAR/QSPR; pp. 1–31.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

