Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry
- PMID: 27529227
- PMCID: PMC5000713
- DOI: 10.3390/ijms17081316
Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry
Abstract
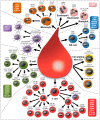
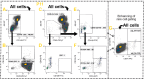
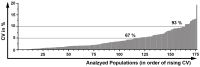
The monitoring of immune cells gained great significance in prognosis and prediction of therapy responses. For analyzing blood samples, the multicolor flow cytometry has become the method of choice as it combines high specificity on single cell level with multiple parameters and high throughput. Here, we present a modular assay for the detailed immunophenotyping of blood (DIoB) that was optimized for an easy and direct application in whole blood samples. The DIoB assay characterizes 34 immune cell subsets that circulate the peripheral blood including all major immune cells such as T cells, B cells, natural killer (NK) cells, monocytes, dendritic cells (DCs), neutrophils, eosinophils, and basophils. In addition, it evaluates their functional state and a few non-leukocytes that also have been associated with the outcome of cancer therapy. This DIoB assay allows a longitudinal and close-meshed monitoring of a detailed immune status in patients requiring only 2.0 mL of peripheral blood and it is not restricted to peripheral blood mononuclear cells. It is currently applied for the immune monitoring of patients with glioblastoma multiforme (IMMO-GLIO-01 trial, NCT02022384), pancreatic cancer (CONKO-007 trial, NCT01827553), and head and neck cancer (DIREKHT trial, NCT02528955) and might pave the way for immune biomarker identification for prediction and prognosis of therapy outcome.
Keywords: adaptive immune system; immune monitoring; immunophenotyping; innate immune system; liquid biopsy; multicolor flow cytometry; whole blood.
Figures






Similar articles
-
Deep phenotyping of immune cell populations by optimized and standardized flow cytometry analyses.Cytometry A. 2018 Aug;93(8):793-802. doi: 10.1002/cyto.a.23570. Epub 2018 Aug 31. Cytometry A. 2018. PMID: 30168890
-
Clinical applications of monitoring immune status with 90 immune cell subsets in human whole blood by 10-color flow cytometry.Int J Lab Hematol. 2021 Oct;43(5):1132-1144. doi: 10.1111/ijlh.13541. Epub 2021 Apr 18. Int J Lab Hematol. 2021. PMID: 33870648
-
OMIP-112: 42-Parameter (40-Color) Spectral Flow Cytometry Panel for Comprehensive Immunophenotyping of Human Peripheral Blood Leukocytes.Cytometry A. 2025 Apr;107(4):226-232. doi: 10.1002/cyto.a.24927. Epub 2025 Mar 17. Cytometry A. 2025. PMID: 40095400
-
Innate cell communication kick-starts pathogen-specific immunity.Nat Immunol. 2016 Apr;17(4):356-63. doi: 10.1038/ni.3375. Nat Immunol. 2016. PMID: 27002843 Free PMC article. Review.
-
An inter-laboratory retrospective analysis of immunotoxicological endpoints in non-human primates: flow cytometry immunophenotyping.J Immunotoxicol. 2013 Oct-Dec;10(4):361-72. doi: 10.3109/1547691X.2012.755237. Epub 2013 Feb 6. J Immunotoxicol. 2013. PMID: 23384295 Review.
Cited by
-
Immune Response Failure in Paucisymptomatic Long-Standing SARS-CoV-2 Spreaders.Clin Pract. 2021 Mar 1;11(1):151-161. doi: 10.3390/clinpract11010021. Clin Pract. 2021. PMID: 33804326 Free PMC article.
-
Immunotherapy: A new target for cancer cure (Review).Oncol Rep. 2023 May;49(5):100. doi: 10.3892/or.2023.8537. Epub 2023 Mar 31. Oncol Rep. 2023. PMID: 36999633 Free PMC article. Review.
-
Effects of serial radon spa therapy on pain and peripheral immune status in patients suffering from musculoskeletal disorders- results from a prospective, randomized, placebo-controlled trial.Front Immunol. 2024 Feb 6;15:1307769. doi: 10.3389/fimmu.2024.1307769. eCollection 2024. Front Immunol. 2024. PMID: 38380316 Free PMC article. Clinical Trial.
-
Treatment with Helicobacter pylori-derived VacA attenuates allergic airway disease.Front Immunol. 2023 Jan 24;14:1092801. doi: 10.3389/fimmu.2023.1092801. eCollection 2023. Front Immunol. 2023. PMID: 36761723 Free PMC article.
-
Distinct Expression Patterns of Interleukin-22 Receptor 1 on Blood Hematopoietic Cells in SARS-CoV-2 Infection.Front Immunol. 2022 Mar 29;13:769839. doi: 10.3389/fimmu.2022.769839. eCollection 2022. Front Immunol. 2022. PMID: 35422799 Free PMC article.
References
-
- Galon J., Angell H.K., Bedognetti D., Marincola F.M. The continuum of cancer immunosurveillance: Prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. - PubMed
-
- Balermpas P., Rodel F., Liberz R., Oppermann J., Wagenblast J., Ghanaati S., Harter P.N., Mittelbronn M., Weiss C., Rodel C., et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br. J. Cancer. 2014;111:1509–1518. doi: 10.1038/bjc.2014.446. - DOI - PMC - PubMed
-
- Ordonez R., Henriquez-Hernandez L.A., Federico M., Valenciano A., Pinar B., Lloret M., Bordon E., Rodriguez-Gallego C., Lara P.C. Radio-induced apoptosis of peripheral blood CD8 T lymphocytes is a novel prognostic factor for survival in cervical carcinoma patients. Strahlenther. Onkol. 2014;190:210–216. doi: 10.1007/s00066-013-0488-x. - DOI - PubMed
-
- Persa E., Balogh A., Safrany G., Lumniczky K. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett. 2015;368:252–261. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

