G Protein-Coupled Receptors in Cancer
- PMID: 27529230
- PMCID: PMC5000717
- DOI: 10.3390/ijms17081320
G Protein-Coupled Receptors in Cancer
Abstract
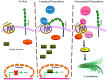
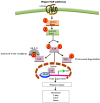
Despite the fact that G protein-coupled receptors (GPCRs) are the largest signal-conveying receptor family and mediate many physiological processes, their role in tumor biology is underappreciated. Numerous lines of evidence now associate GPCRs and their downstream signaling targets in cancer growth and development. Indeed, GPCRs control many features of tumorigenesis, including immune cell-mediated functions, proliferation, invasion and survival at the secondary site. Technological advances have further substantiated GPCR modifications in human tumors. Among these are point mutations, gene overexpression, GPCR silencing by promoter methylation and the number of gene copies. At this point, it is imperative to elucidate specific signaling pathways of "cancer driver" GPCRs. Emerging data on GPCR biology point to functional selectivity and "biased agonism"; hence, there is a diminishing enthusiasm for the concept of "one drug per GPCR target" and increasing interest in the identification of several drug options. Therefore, determining the appropriate context-dependent conformation of a functional GPCR as well as the contribution of GPCR alterations to cancer development remain significant challenges for the discovery of dominant cancer genes and the development of targeted therapeutics.
Keywords: CXCR4; G protein-coupled receptors (GPCRs); Hippo/YAP; LPA(1-6); PH-domain; Wnt/β-catenin; cancer; oncogenes; protease; protease-activated receptor; protease-activated receptors (PARs).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

