Protective Effects of Berberine on Renal Injury in Streptozotocin (STZ)-Induced Diabetic Mice
- PMID: 27529235
- PMCID: PMC5000724
- DOI: 10.3390/ijms17081327
Protective Effects of Berberine on Renal Injury in Streptozotocin (STZ)-Induced Diabetic Mice
Abstract
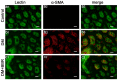
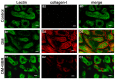
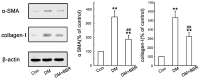
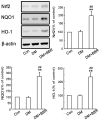
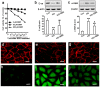
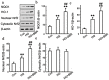
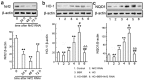
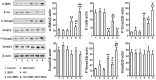
Diabetic nephropathy (DN) is a serious diabetic complication with renal hypertrophy and expansion of extracellular matrices in renal fibrosis. Epithelial-to-mesenchymal transition (EMT) of renal tubular epithelial cells may be involved in the main mechanism. Berberine (BBR) has been shown to have antifibrotic effects in liver, kidney and lung. However, the mechanism of cytoprotective effects of BBR in DN is still unclear. In this study, we investigated the curative effects of BBR on tubulointerstitial fibrosis in streptozotocin (STZ)-induced diabetic mice and the high glucose (HG)-induced EMT in NRK 52E cells. We found that BBR treatment attenuated renal fibrosis by activating the nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling pathway in the diabetic kidneys. Further revealed that BBR abrogated HG-induced EMT and oxidative stress in relation not only with the activation of Nrf2 and two Nrf2-targeted antioxidative genes (NQO-1 and HO-1), but also with the suppressing the activation of TGF-β/Smad signaling pathway. Importantly, knockdown Nrf2 with siRNA not only abolished the BBR-induced expression of HO-1 and NQO-1 but also removed the inhibitory effect of BBR on HG-induced activation of TGF-β/Smad signaling as well as the anti-fibrosis effects. The data from present study suggest that BBR can ameliorate tubulointerstitial fibrosis in DN by activating Nrf2 pathway and inhibiting TGF-β/Smad/EMT signaling activity.
Keywords: EMT; Nrf2 pathway; TGF-β/Smad signaling pathway; berberbine; diabetic nephropathy; renal tubular epithelial cells.
Figures
Similar articles
-
Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the Notch/snail pathway in diabetic nephropathy model KKAy mice.Drug Des Devel Ther. 2017 Mar 31;11:1065-1079. doi: 10.2147/DDDT.S124971. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28408805 Free PMC article.
-
Attenuation of diabetic nephropathy by Sanziguben Granule inhibiting EMT through Nrf2-mediated anti-oxidative effects in streptozotocin (STZ)-induced diabetic rats.J Ethnopharmacol. 2017 Jun 9;205:207-216. doi: 10.1016/j.jep.2017.05.009. Epub 2017 May 10. J Ethnopharmacol. 2017. PMID: 28501426
-
A bioinformatics and network pharmacology approach to the mechanisms of action of Shenxiao decoction for the treatment of diabetic nephropathy.Phytomedicine. 2020 Apr;69:153192. doi: 10.1016/j.phymed.2020.153192. Epub 2020 Feb 22. Phytomedicine. 2020. PMID: 32200292
-
Berberine as a promising anti-diabetic nephropathy drug: An analysis of its effects and mechanisms.Eur J Pharmacol. 2015 Aug 5;760:103-12. doi: 10.1016/j.ejphar.2015.04.017. Epub 2015 Apr 24. Eur J Pharmacol. 2015. PMID: 25912800 Review.
-
Protective effects of berberine on various kidney diseases: Emphasis on the promising effects and the underlined molecular mechanisms.Life Sci. 2022 Oct 1;306:120697. doi: 10.1016/j.lfs.2022.120697. Epub 2022 Jun 16. Life Sci. 2022. PMID: 35718235 Review.
Cited by
-
Berberine Reduces Lipid Accumulation by Promoting Fatty Acid Oxidation in Renal Tubular Epithelial Cells of the Diabetic Kidney.Front Pharmacol. 2022 Jan 5;12:729384. doi: 10.3389/fphar.2021.729384. eCollection 2021. Front Pharmacol. 2022. PMID: 35069186 Free PMC article.
-
Acteoside-containing caffeic acid is bioactive functional group of antifibrotic effect by suppressing inflammation via inhibiting AHR nuclear translocation in chronic kidney disease.Acta Pharmacol Sin. 2025 Jun 20. doi: 10.1038/s41401-025-01598-4. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40542283
-
Time-dose response and mechanistic specificity of berberine in renal fibrosis from a multi-model integration perspective: a systematic review and meta-analysis on animal models.Front Pharmacol. 2025 Jun 11;16:1600408. doi: 10.3389/fphar.2025.1600408. eCollection 2025. Front Pharmacol. 2025. PMID: 40567371 Free PMC article.
-
Advances in the pharmacological mechanisms of berberine in the treatment of fibrosis.Front Pharmacol. 2024 Sep 20;15:1455058. doi: 10.3389/fphar.2024.1455058. eCollection 2024. Front Pharmacol. 2024. PMID: 39372209 Free PMC article. Review.
-
Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway.Biol Res. 2018 Mar 31;51(1):9. doi: 10.1186/s40659-018-0157-8. Biol Res. 2018. PMID: 29604956 Free PMC article.
References
-
- Gilbert R.E., Cox A., Wu L.L., Allen T.J., Hulthen U.L., Jerums G., Cooper M.E. Expression of transforming growth factor-beta1 and type IV collagen in the renal tubulointerstitium in experimental diabetes: Effects of ACE inhibition. Diabetes. 1998;47:414–422. doi: 10.2337/diabetes.47.3.414. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

