The Role of Flavonoids in Nodulation Host-Range Specificity: An Update
- PMID: 27529286
- PMCID: PMC5039741
- DOI: 10.3390/plants5030033
The Role of Flavonoids in Nodulation Host-Range Specificity: An Update
Abstract
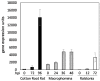
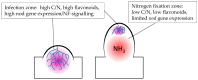
Flavonoids are crucial signaling molecules in the symbiosis between legumes and their nitrogen-fixing symbionts, the rhizobia. The primary function of flavonoids in the interaction is to induce transcription of the genes for biosynthesis of the rhizobial signaling molecules called Nod factors, which are perceived by the plant to allow symbiotic infection of the root. Many legumes produce specific flavonoids that only induce Nod factor production in homologous rhizobia, and therefore act as important determinants of host range. Despite a wealth of evidence on legume flavonoids, relatively few have proven roles in rhizobial infection. Recent studies suggest that production of key "infection" flavonoids is highly localized at infection sites. Furthermore, some of the flavonoids being produced at infection sites are phytoalexins and may have a role in the selection of compatible symbionts during infection. The molecular details of how flavonoid production in plants is regulated during nodulation have not yet been clarified, but nitrogen availability has been shown to play a role.
Keywords: daidzein; genistein; medicarpin; methoxychalcone; phytoalexins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Regulation of Rhizobial Nodulation Genes by Flavonoid-Independent NodD Supports Nitrogen-Fixing Symbioses With Legumes.Environ Microbiol. 2025 Jan;27(1):e70014. doi: 10.1111/1462-2920.70014. Environ Microbiol. 2025. PMID: 39865396 Free PMC article.
-
Physiological impact of flavonoids on nodulation and ureide metabolism in legume plants.Plant Physiol Biochem. 2021 Sep;166:512-521. doi: 10.1016/j.plaphy.2021.06.007. Epub 2021 Jun 16. Plant Physiol Biochem. 2021. PMID: 34171572 Review.
-
Rhizobial Exopolysaccharides: Genetic Regulation of Their Synthesis and Relevance in Symbiosis with Legumes.Int J Mol Sci. 2021 Jun 9;22(12):6233. doi: 10.3390/ijms22126233. Int J Mol Sci. 2021. PMID: 34207734 Free PMC article. Review.
-
Nodulation outer proteins: double-edged swords of symbiotic rhizobia.Biochem J. 2015 Sep 15;470(3):263-74. doi: 10.1042/BJ20150518. Biochem J. 2015. PMID: 26341483 Review.
-
A Germin-Like Protein GLP1 of Legumes Mediates Symbiotic Nodulation by Interacting with an Outer Membrane Protein of Rhizobia.Microbiol Spectr. 2023 Feb 14;11(1):e0335022. doi: 10.1128/spectrum.03350-22. Epub 2023 Jan 12. Microbiol Spectr. 2023. PMID: 36633436 Free PMC article.
Cited by
-
Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture.Plants (Basel). 2020 Aug 11;9(8):1011. doi: 10.3390/plants9081011. Plants (Basel). 2020. PMID: 32796519 Free PMC article. Review.
-
Response of Cucumis sativus to Neighbors in a Species-Specific Manner.Plants (Basel). 2022 Dec 27;12(1):139. doi: 10.3390/plants12010139. Plants (Basel). 2022. PMID: 36616268 Free PMC article.
-
Evaluation of qPCR to Detect Shifts in Population Composition of the Rhizobial Symbiont Mesorhizobium japonicum during Serial in Planta Transfers.Biology (Basel). 2023 Feb 9;12(2):277. doi: 10.3390/biology12020277. Biology (Basel). 2023. PMID: 36829553 Free PMC article.
-
A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin.Int J Mol Sci. 2023 Feb 7;24(4):3266. doi: 10.3390/ijms24043266. Int J Mol Sci. 2023. PMID: 36834673 Free PMC article. Review.
-
Chromium(VI) Toxicity in Legume Plants: Modulation Effects of Rhizobial Symbiosis.Biomed Res Int. 2018 Feb 14;2018:8031213. doi: 10.1155/2018/8031213. eCollection 2018. Biomed Res Int. 2018. PMID: 29662899 Free PMC article. Review.
References
-
- Spaink H.P., Sheeley D.M., Vanbrussel A.A.N., Glushka J., York W.S., Tak T., Geiger O., Kennedy E.P., Reinhold V.N., Lugtenberg B.J.J. A novel highly unsaturated fatty-acid moiety of lipo-oligosaccharide signals determines host specificity of rhizobium. Nature. 1991;354:125–130. doi: 10.1038/354125a0. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

