MicroRNA-200c Represses IL-6, IL-8, and CCL-5 Expression and Enhances Osteogenic Differentiation
- PMID: 27529418
- PMCID: PMC4987006
- DOI: 10.1371/journal.pone.0160915
MicroRNA-200c Represses IL-6, IL-8, and CCL-5 Expression and Enhances Osteogenic Differentiation
Erratum in
-
Correction: MicroRNA-200c Represses IL-6, IL-8, and CCL-5 Expression and Enhances Osteogenic Differentiation.PLoS One. 2016 Dec 29;11(12):e0169381. doi: 10.1371/journal.pone.0169381. eCollection 2016. PLoS One. 2016. PMID: 28033413 Free PMC article.
Abstract
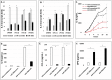
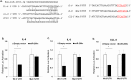
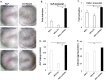
MicroRNAs (miRs) regulate inflammation and BMP antagonists, thus they have potential uses as therapeutic reagents. However, the molecular function of miR-200c in modulating proinflammatory and bone metabolic mediators and osteogenic differentiation is not known. After miR-200c was transduced into a human embryonic palatal mesenchyme (HEPM) (a cell line of preosteoblasts), using lentiviral vectors, the resulting miR-200c overexpression increased osteogenic differentiation biomarkers, including osteocalcin (OCN) transcripts and calcium content. miR-200c expression also down-regulated interleukin (IL)-6, IL-8, and chemokine (C-C motif) ligand (CCL)-5 under lipopolysaccharide (LPS) stimulation and increased osteoprotegerin (OPG) in these cells. miR-200c directly regulates the expression of IL-6, IL-8 and CCL-5 transcripts by binding to their 3'UTRs. A plasmid-based miR-200c inhibitor effectively reduces their binding activities. Additionally, miR-200c delivered using polyethylenimine (PEI) nanoparticles effectively inhibits IL-6, IL-8 and CCL-5 in primary human periodontal ligament fibroblasts and increases the biomarkers of osteogenic differentiation in human bone marrow mesenchymal stem cells (MSCs), including calcium content, ALP, and Runx2. These data demonstrate that miR-200c represses IL-6, IL-8 and CCL-5 and improves osteogenic differentiation. miR-200c may potentially be used as an effective means to prevent periodontitis-associated bone loss by arresting inflammation and osteoclastogenesis and enhancing bone regeneration.
Conflict of interest statement
Figures





Similar articles
-
MicroRNA-200c Attenuates Periodontitis by Modulating Proinflammatory and Osteoclastogenic Mediators.Stem Cells Dev. 2019 Aug 1;28(15):1026-1036. doi: 10.1089/scd.2019.0027. Epub 2019 May 20. Stem Cells Dev. 2019. PMID: 31017046 Free PMC article.
-
MicroRNA-200c promotes osteogenic differentiation of human bone mesenchymal stem cells through activating the AKT/β-Catenin signaling pathway via downregulating Myd88.J Cell Physiol. 2019 Dec;234(12):22675-22686. doi: 10.1002/jcp.28834. Epub 2019 May 31. J Cell Physiol. 2019. PMID: 31152447
-
[Human bone marrow mesenchymal stem cell exosome-derived miR-335-5p promotes osteogenic differentiation of human periodontal ligament stem cells to alleviate periodontitis by downregulating DKK1].Nan Fang Yi Ke Da Xue Xue Bao. 2023 Mar 20;43(3):420-427. doi: 10.12122/j.issn.1673-4254.2023.03.12. Nan Fang Yi Ke Da Xue Xue Bao. 2023. PMID: 37087587 Free PMC article. Chinese.
-
miR-206 inhibits osteogenic differentiation of bone marrow mesenchymal stem cells by targetting glutaminase.Biosci Rep. 2019 Mar 26;39(3):BSR20181108. doi: 10.1042/BSR20181108. Print 2019 Mar 29. Biosci Rep. 2019. PMID: 30804229 Free PMC article.
-
Enhancement of MicroRNA-200c on Osteogenic Differentiation and Bone Regeneration by Targeting Sox2-Mediated Wnt Signaling and Klf4.Hum Gene Ther. 2019 Nov;30(11):1405-1418. doi: 10.1089/hum.2019.019. Epub 2019 Aug 16. Hum Gene Ther. 2019. PMID: 31288577 Free PMC article.
Cited by
-
MicroRNA-200c Attenuates Periodontitis by Modulating Proinflammatory and Osteoclastogenic Mediators.Stem Cells Dev. 2019 Aug 1;28(15):1026-1036. doi: 10.1089/scd.2019.0027. Epub 2019 May 20. Stem Cells Dev. 2019. PMID: 31017046 Free PMC article.
-
CaCO3 Nanoparticles Delivering MicroRNA-200c Suppress Oral Squamous Cell Carcinoma.J Dent Res. 2024 Feb;103(2):147-155. doi: 10.1177/00220345231216110. Epub 2023 Dec 27. J Dent Res. 2024. PMID: 38149503 Free PMC article.
-
MicroRNA Profiling in Cartilage Ageing.Int J Genomics. 2017;2017:2713725. doi: 10.1155/2017/2713725. Epub 2017 Aug 14. Int J Genomics. 2017. PMID: 28890892 Free PMC article.
-
The role of long non-coding RNA (lncRNA) nuclear paraspeckle assembly transcript 1 (NEAT1) in chronic periodontitis progression.Bioengineered. 2022 Feb;13(2):2336-2345. doi: 10.1080/21655979.2021.2018387. Bioengineered. 2022. PMID: 35034548 Free PMC article.
-
miR-140 inhibits osteogenic differentiation of human periodontal ligament fibroblasts through ras homolog gene family, member A -transcriptional co-activator with PDZ-binding motif pathway.Kaohsiung J Med Sci. 2021 Jan;37(1):38-46. doi: 10.1002/kjm2.12293. Epub 2020 Aug 25. Kaohsiung J Med Sci. 2021. PMID: 32841515 Free PMC article.
References
-
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012; 91: 914–1920. - PubMed
-
- Papapanou PN, Tonetti MS. Diagnosis and epidemiology of periodontal osseous lesions. Periodontol. 2000; 22: 8–21. - PubMed
-
- Müller HP, Ulbrich M. Alveolar bone levels in adults as assessed on panoramic radiographs. (I) Prevalence, extent, and severity of even and angular bone loss. Clin Oral Investig. 2005; 9: 98–104 - PubMed
-
- Armas J, Culshaw S, Savarrio L. Treatment of peri-implant diseases: a review of the literature and protocol proposal. Dent Update. 2013; 40:472–480. - PubMed
-
- Jeffcoat MK. Osteoporosis: a possible modifying factor in oral bone loss. Ann Periodontol. 1998; 3: 312–321 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

