Therapeutic interactions between mesenchymal stem cells for healing medication-related osteonecrosis of the jaw
- PMID: 27530108
- PMCID: PMC4988021
- DOI: 10.1186/s13287-016-0367-3
Therapeutic interactions between mesenchymal stem cells for healing medication-related osteonecrosis of the jaw
Abstract
Background: Mesenchymal stem cells (MSCs) have been isolated from a variety of tissues, including bone marrow, adipose, and mucosa. MSCs have the capacity for self-renewal and differentiation. Reports have been published on the systemic administration of MSCs leading to functional improvements by engraftment and differentiation, thus providing a new strategy to regenerate damaged tissues. Recently, it has become clear that MSCs possess immunomodulatory properties and can therefore be used to treat diseases. However, the therapeutic effect mechanisms of MSCs are yet to be determined. Here, we investigated these mechanisms using a medication-related osteonecrosis of the jaw (MRONJ)-like mouse model.
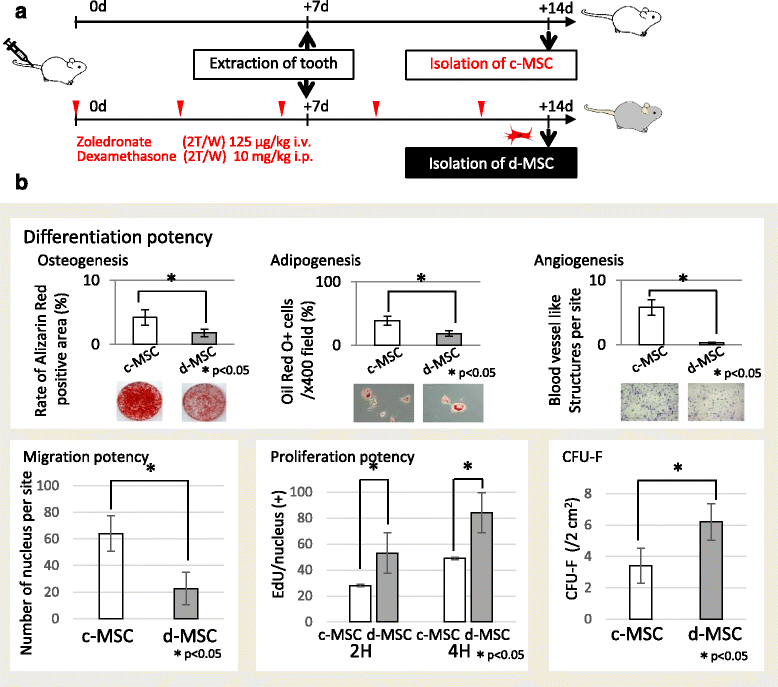
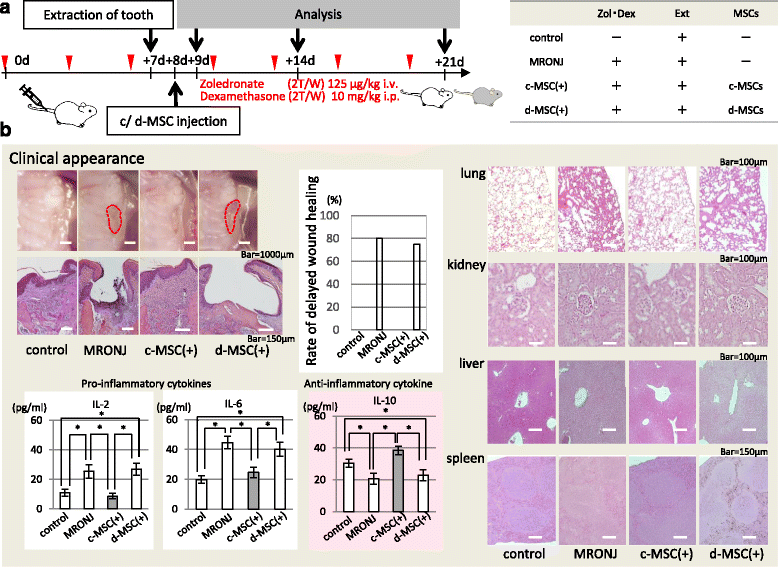
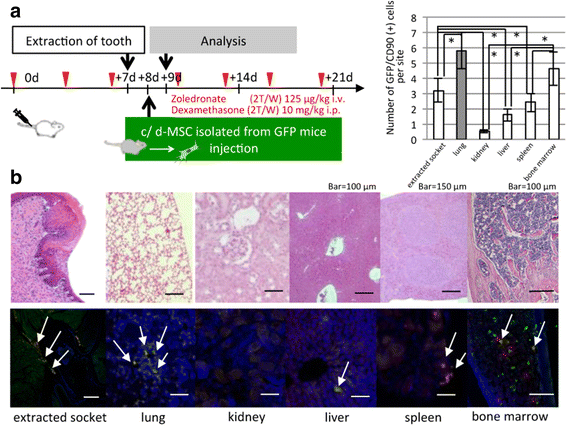
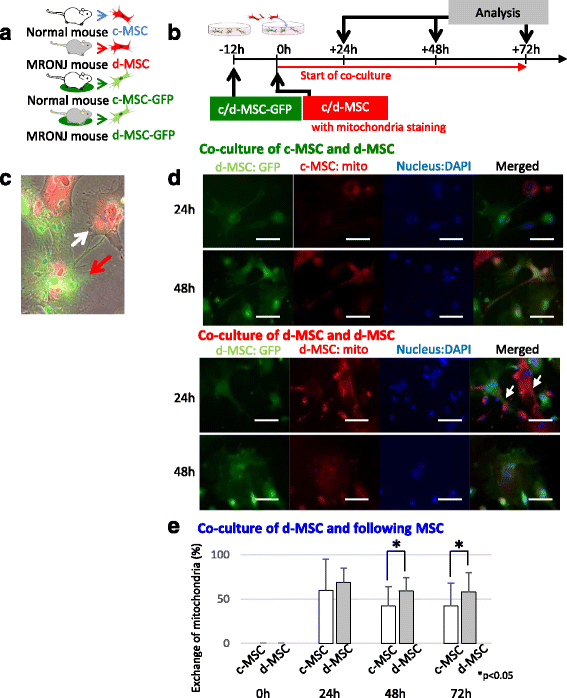
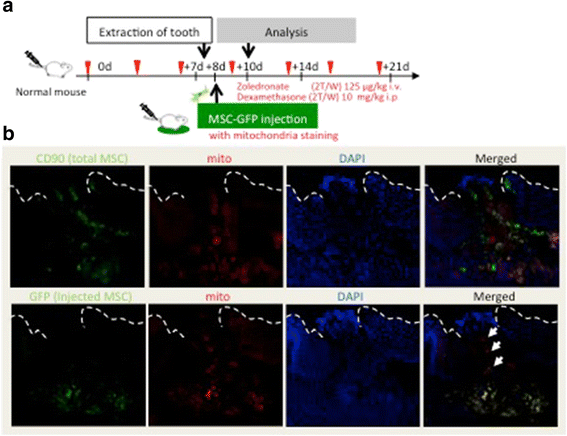
Methods: To generate MRONJ-like characteristics, mice received intravenous zoledronate and dexamethasone two times a week. At 1 week after intravenous injection, maxillary first molars were extracted, and at 1 week after tooth extraction, MSCs were isolated from the bone marrow of the mice femurs and tibias. To compare "diseased MSCs" from MRONJ-like mice (d-MSCs) with "control MSCs" from untreated mice (c-MSCs), the isolated MSCs were analyzed by differentiation and colony-forming unit-fibroblast (CFU-F) assays and systemic transplantation of either d-MSCs or c-MSCs into MRONJ-like mice. Furthermore, we observed the exchange of cell contents among d-MSCs and c-MSCs during coculture with all combinations of each MSC type.
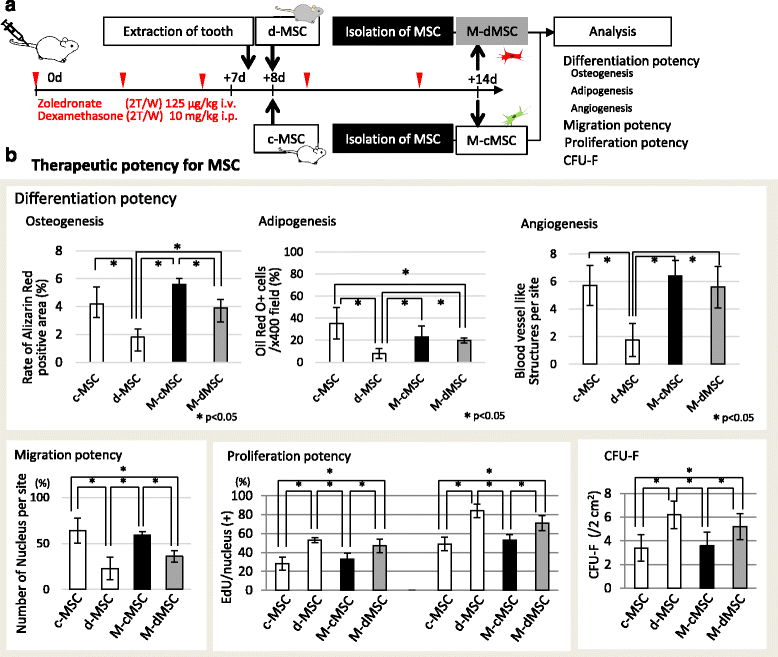
Results: d-MSCs were inferior to c-MSCs in differentiation and CFU-F assays. Moreover, the d-MSC-treated group did not show earlier healing in MRONJ-like mice. In cocultures with any combination, MSC pairs formed cell-cell contacts and exchanged cell contents. Interestingly, the exchange among c-MSCs and d-MSCs was more frequently observed than other pairs, and d-MSCs were distinguishable from c-MSCs.
Conclusions: The interaction of c-MSCs and d-MSCs, including exchange of cell contents, contributes to the treatment potential of d-MSCs. This cellular behavior might be one therapeutic mechanism used by MSCs for MRONJ.
Keywords: Medication-related osteonecrosis of the jaw; Mesenchymal stem cell; Therapeutic mechanism.
Figures
Similar articles
-
Mesenchymal stem cells in the treatment of osteonecrosis of the jaw.J Korean Assoc Oral Maxillofac Surg. 2021 Apr 30;47(2):65-75. doi: 10.5125/jkaoms.2021.47.2.65. J Korean Assoc Oral Maxillofac Surg. 2021. PMID: 33911038 Free PMC article. Review.
-
Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model.Acta Biomater. 2016 Sep 15;42:400-410. doi: 10.1016/j.actbio.2016.06.022. Epub 2016 Jun 17. Acta Biomater. 2016. PMID: 27326918
-
[Age-related biological characteristics of human bone marrow mesenchymal stem cells from different age donors].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005 Dec;13(6):1049-53. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005. PMID: 16403278 Chinese.
-
Paracrine action of mesenchymal stromal cells delivered by microspheres contributes to cutaneous wound healing and prevents scar formation in mice.Cytotherapy. 2015 Jul;17(7):922-31. doi: 10.1016/j.jcyt.2015.03.690. Epub 2015 May 1. Cytotherapy. 2015. PMID: 25939802
-
Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications.Arch Pharm Res. 2012 Feb;35(2):213-21. doi: 10.1007/s12272-012-0202-z. Epub 2012 Feb 28. Arch Pharm Res. 2012. PMID: 22370776 Review.
Cited by
-
Revolutionizing bone defect healing: the power of mesenchymal stem cells as seeds.Front Bioeng Biotechnol. 2024 Oct 21;12:1421674. doi: 10.3389/fbioe.2024.1421674. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39497791 Free PMC article. Review.
-
Oral fibroblasts rescue osteogenic differentiation of mesenchymal stem cells after exposure to Zoledronic acid in a paracrine effect.Front Pharmacol. 2023 Aug 11;14:1172705. doi: 10.3389/fphar.2023.1172705. eCollection 2023. Front Pharmacol. 2023. PMID: 37637413 Free PMC article.
-
Exosomes from Adipose-Derived Mesenchymal Stromal Cells Prevent Medication-Related Osteonecrosis of the Jaw by Inhibiting Macrophage M1 Polarization and Pyroptosis.Int J Nanomedicine. 2024 Nov 26;19:12675-12693. doi: 10.2147/IJN.S482849. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39619056 Free PMC article.
-
Preclinical models of medication-related osteonecrosis of the jaw (MRONJ).Bone. 2021 Dec;153:116184. doi: 10.1016/j.bone.2021.116184. Epub 2021 Sep 11. Bone. 2021. PMID: 34520898 Free PMC article. Review.
-
Mesenchymal stem cells in the treatment of osteonecrosis of the jaw.J Korean Assoc Oral Maxillofac Surg. 2021 Apr 30;47(2):65-75. doi: 10.5125/jkaoms.2021.47.2.65. J Korean Assoc Oral Maxillofac Surg. 2021. PMID: 33911038 Free PMC article. Review.
References
-
- Edwards BJ, Hellstein JW, Jacobsen PL, Kaltman S, Mariotti A, Migliorati CA, American Dental Association Council on Scientific Affairs Expert Panel on Bisphosphonate-Associated Osteonecrosis of the Jaw Updated recommendations for managing the care of patients receiving oral bisphosphonate therapy: an advisory statement from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2008;139:1674–1677. doi: 10.14219/jada.archive.2008.0110. - DOI - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group. Coleman R, Powles T, Paterson A, Gnant M, Anderson S, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

