Immune tolerance to an intestine-adapted bacteria, Chryseobacterium sp., injected into the hemocoel of Protaetia brevitarsis seulensis
- PMID: 27530146
- PMCID: PMC4987663
- DOI: 10.1038/srep31722
Immune tolerance to an intestine-adapted bacteria, Chryseobacterium sp., injected into the hemocoel of Protaetia brevitarsis seulensis
Abstract
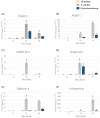
To explore the interaction of gut microbes and the host immune system, bacteria were isolated from the gut of Protaetia brevitarsis seulensis larvae. Chryseobacterium sp., Bacillus subtilis, Arthrobacter arilaitensis, Bacillus amyloliquefaciens, Bacillus megaterium, and Lysinibacillus xylanilyticus were cultured in vitro, identified, and injected in the hemocoel of P. brevitarsis seulensis larvae, respectively. There were no significant changes in phagocytosis-associated lysosomal formation or pathogen-related autophagosome in immune cells (granulocytes) from Chryseobacterium sp.-challenged larvae. Next, we examined changes in the transcription of innate immune genes such as peptidoglycan recognition proteins and antimicrobial peptides following infection with Chryseobacterium sp. PGRP-1 and -2 transcripts, which may be associated with melanization generated by prophenoloxidase (PPO), were either highly or moderately expressed at 24 h post-infection with Chryseobacterium sp. However, PGRP-SC2 transcripts, which code for bactericidal amidases, were expressed at low levels. With respect to antimicrobial peptides, only coleoptericin was moderately expressed in Chryseobacterium sp.-challenged larvae, suggesting maintenance of an optimum number of Chryseobacterium sp. All examined genes were expressed at significantly higher levels in larvae challenged with a pathogenic bacterium. Our data demonstrated that gut-inhabiting bacteria, the Chryseobacterium sp., induced a weaker immune response than other pathogenic bacteria, E. coli K12.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

