Edoxaban drug-drug interactions with ketoconazole, erythromycin, and cyclosporine
- PMID: 27530188
- PMCID: PMC5099547
- DOI: 10.1111/bcp.13092
Edoxaban drug-drug interactions with ketoconazole, erythromycin, and cyclosporine
Abstract
Aims: Edoxaban, a novel factor Xa inhibitor, is a substrate of cytochrome P450 3 A4 (CYP3A4) and the efflux transporter P-glycoprotein (P-gp). Three edoxaban drug-drug interaction studies examined the effects of P-gp inhibitors with varying degrees of CYP3A4 inhibition.
Methods: In each study, healthy subjects received a single oral dose of 60 mg edoxaban with or without an oral dual P-gp/CYP3A4 inhibitor as follows: ketoconazole 400 mg once daily for 7 days, edoxaban on day 4; erythromycin 500 mg four times daily for 8 days, edoxaban on day 7; or single dose of cyclosporine 500 mg with edoxaban. Serial plasma samples were obtained for pharmacokinetics and pharmacodynamics. Safety was assessed throughout the study.
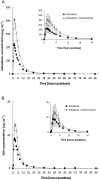
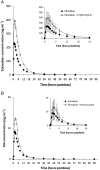
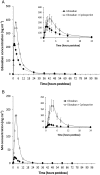
Results: Coadministration of ketoconazole, erythromycin, or cyclosporine increased edoxaban total exposure by 87%, 85%, and 73%, respectively, and the peak concentration by 89%, 68%, and 74%, respectively, compared with edoxaban alone. The half-life did not change appreciably. Exposure of M4, the major active edoxaban metabolite, was consistent when edoxaban was administered alone or with ketoconazole and erythromycin. With cyclosporine, M4 total exposure increased by 6.9-fold and peak exposure by 8.7-fold, suggesting an additional interaction. Pharmacodynamic effects were reflective of increased edoxaban exposure. No clinically significant adverse events were observed.
Conclusions: Administration of dual inhibitors of P-gp and CYP3A4 increased edoxaban exposure by less than two-fold. This effect appears to be primarily due to inhibition of P-gp. The impact of CYP3A4 inhibition appears to be less pronounced, and its contribution to total clearance appears limited in healthy subjects.
Keywords: CYP3A4; P-glycoprotein; drug interactions; edoxaban; pharmacokinetics.
© 2016 Daiichi Sankyo. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures

References
-
- Furugohri T, Isobe K, Honda Y, Kamisato‐Matsumoto C, Sugiyama N, Nagahara T, et al. DU‐176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost 2008; 6: 1542–1549. - PubMed
-
- SAVAYSA . (Edoxaban) Tablets for Oral Use. Highlights of Prescribing Information. Daiichi Sankyo Co: Parsippany, NJ, USA, 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

