Serum pharmacodynamic biomarkers for chronic corticosteroid treatment of children
- PMID: 27530235
- PMCID: PMC4987691
- DOI: 10.1038/srep31727
Serum pharmacodynamic biomarkers for chronic corticosteroid treatment of children
Abstract
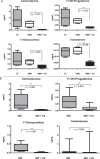
Corticosteroids are extensively used in pediatrics, yet the burden of side effects is significant. Availability of a simple, fast, and reliable biochemical read out of steroidal drug pharmacodynamics could enable a rapid and objective assessment of safety and efficacy of corticosteroids and aid development of corticosteroid replacement drugs. To identify potential corticosteroid responsive biomarkers we performed proteome profiling of serum samples from DMD and IBD patients with and without corticosteroid treatment using SOMAscan aptamer panel testing 1,129 proteins in <0.1 cc of sera. Ten pro-inflammatory proteins were elevated in untreated patients and suppressed by corticosteroids (MMP12, IL22RA2, CCL22, IGFBP2, FCER2, LY9, ITGa1/b1, LTa1/b2, ANGPT2 and FGG). These are candidate biomarkers for anti-inflammatory efficacy of corticosteroids. Known safety concerns were validated, including elevated non-fasting insulin (insulin resistance), and elevated angiotensinogen (salt retention). These were extended by new candidates for metabolism disturbances (leptin, afamin), stunting of growth (growth hormone binding protein), and connective tissue remodeling (MMP3). Significant suppression of multiple adrenal steroid hormones was also seen in treated children (reductions of 17-hydroxyprogesterone, corticosterone, 11-deoxycortisol and testosterone). A panel of new pharmacodynamic biomarkers for corticosteroids in children was defined. Future studies will need to bridge specific biomarkers to mechanism of drug action, and specific clinical outcomes.
Figures


References
-
- Hillier S. G. Diamonds are forever: the cortisone legacy. The Journal of endocrinology 195, 1–6 (2007). - PubMed
-
- Swartz S. L. & Dluhy R. G. Corticosteroids: clinical pharmacology and therapeutic use. Drugs 16, 238–255 (1978). - PubMed
-
- Kapadia C. R. et al.. Endocrine Effects of Inhaled Corticosteroids in Children. JAMA Pediatr 170, 163–170 (2016). - PubMed
-
- von Scheven E., Corbin K. J., Stagi S. & Cimaz R. Glucocorticoid-associated osteoporosis in chronic inflammatory diseases: epidemiology, mechanisms, diagnosis, and treatment. Curr Osteoporos Rep 12, 289–299 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

