Characterization of VHL missense mutations in sporadic clear cell renal cell carcinoma: hotspots, affected binding domains, functional impact on pVHL and therapeutic relevance
- PMID: 27530247
- PMCID: PMC4987997
- DOI: 10.1186/s12885-016-2688-0
Characterization of VHL missense mutations in sporadic clear cell renal cell carcinoma: hotspots, affected binding domains, functional impact on pVHL and therapeutic relevance
Abstract
Background: The VHL protein (pVHL) is a multiadaptor protein that interacts with more than 30 different binding partners involved in many oncogenic processes. About 70 % of clear cell renal cell carcinoma (ccRCC) have VHL mutations with varying impact on pVHL function. Loss of pVHL function leads to the accumulation of Hypoxia Inducible Factor (HIF), which is targeted by current targeted treatments. In contrast to nonsense and frameshift mutations that highly likely nullify pVHL multipurpose functions, missense mutations may rather specifically influence the binding capability of pVHL to its partners. The affected pathways may offer predictive clues to therapy and response to treatment. In this study we focused on the VHL missense mutation pattern in ccRCC, and studied their potential effects on pVHL protein stability and binding partners and discussed treatment options.
Methods: We sequenced VHL in 360 sporadic ccRCC FFPE samples and compared observed and expected frequency of missense mutations in 32 different binding domains. The prediction of the impact of those mutations on protein stability and function was assessed in silico. The response to HIF-related, anti-angiogenic treatment of 30 patients with known VHL mutation status was also investigated.
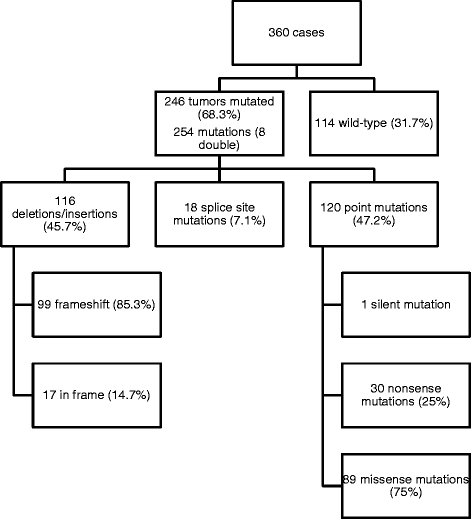
Results: We identified 254 VHL mutations (68.3 % of the cases) including 89 missense mutations (35 %). Codons Ser65, Asn78, Ser80, Trp117 and Leu184 represented hotspots and missense mutations in Trp117 and Leu 184 were predicted to highly destabilize pVHL. About 40 % of VHL missense mutations were predicted to cause severe protein malfunction. The pVHL binding domains for HIF1AN, BCL2L11, HIF1/2α, RPB1, PRKCZ, aPKC-λ/ι, EEF1A1, CCT-ζ-2, and Cullin2 were preferentially affected. These binding partners are mainly acting in transcriptional regulation, apoptosis and ubiquitin ligation. There was no correlation between VHL mutation status and response to treatment.
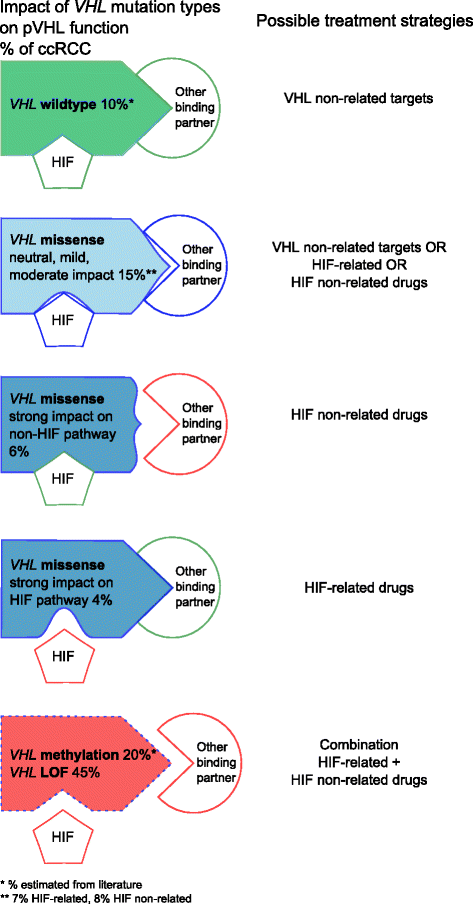
Conclusions: VHL missense mutations may exert mild, moderate or strong impact on pVHL stability. Besides the HIF binding domain, other pVHL binding sites seem to be non-randomly altered by missense mutations. In contrast to LOF mutations that affect all the different pathways normally controlled by pVHL, missense mutations may be rather appropriate for designing tailor-made treatment strategies for ccRCC.
Keywords: Binding domains; Clear cell renal cell carcinoma; Missense mutations; Therapy; VHL; pVHL stability.
Figures


References
-
- National Cancer Institute. http://seer.cancer.gov/. Accessed 21 Aug 2015.
-
- World Cancer reports, International Agency for Research on Cancer. http://www.iarc.fr/. Accessed 21 Aug 2015.
-
- The World Health Organization. http://www.who.org/. Accessed 21 Aug 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

