Serial changes in the proliferation and differentiation of adipose-derived stem cells after ionizing radiation
- PMID: 27530249
- PMCID: PMC4988041
- DOI: 10.1186/s13287-016-0378-0
Serial changes in the proliferation and differentiation of adipose-derived stem cells after ionizing radiation
Abstract
Background: Adipose-derived stem cells (ASCs) are important to homeostasis and the regeneration of subcutaneous fat. Hence, we examined the proliferation and differentiation capacity of irradiated ASCs over time.
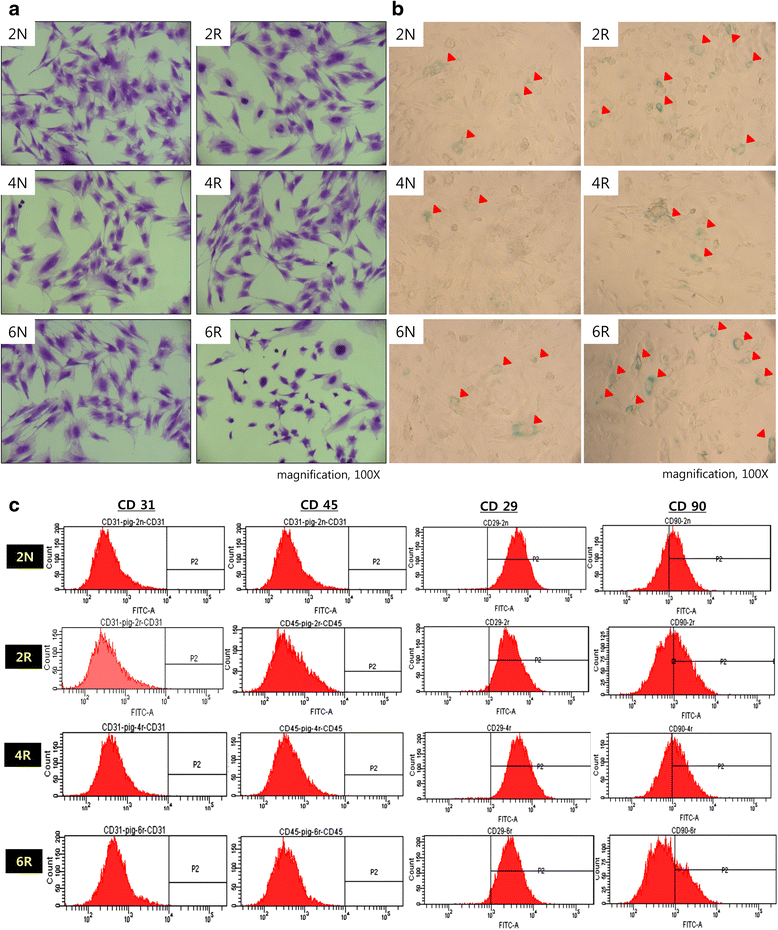
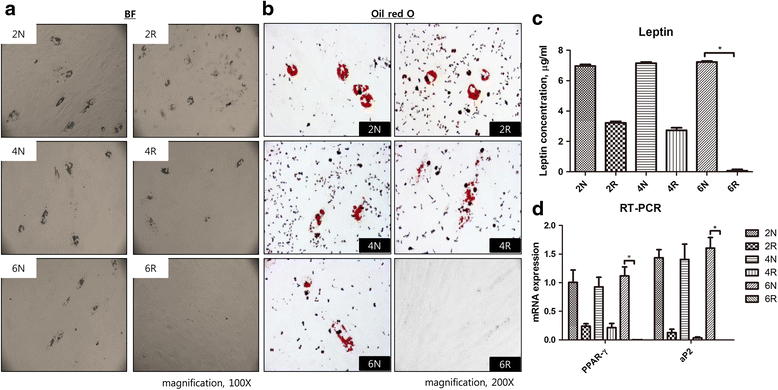
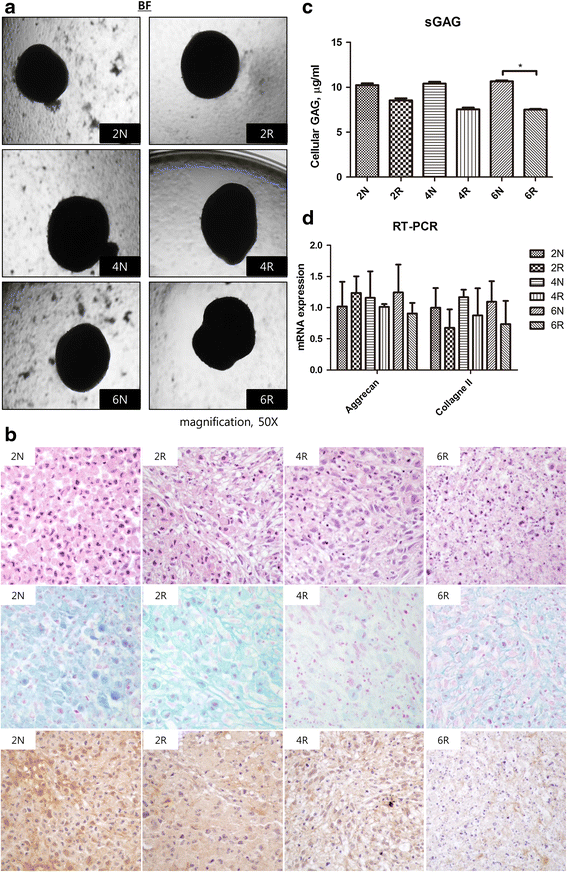
Methods: Two female pigs received a single 18 Gy dose of ionizing radiation to an 18 × 8 cm area on the dorsal body skin via a 6 MeV electron beam. After irradiation, the ASCs were cultured from adipose tissue harvested from a non-irradiated area and an irradiated area at 2, 4, and 6 weeks. The proliferation capacity of ASCs was evaluated by a colony-forming units-fibroblasts (CFUs-Fs) assay, a cholecystokinin (CCK) test with 10 % fetal bovine serum (FBS), and a 1 % FBS culture test. The senescence of ASCs was evaluated through morphological examination, immunophenotyping, and β-galactosidase activity, and the multipotent differentiation potential of ASCs was evaluated in adipogenic, osteogenic, and chondrogenic differentiation media.
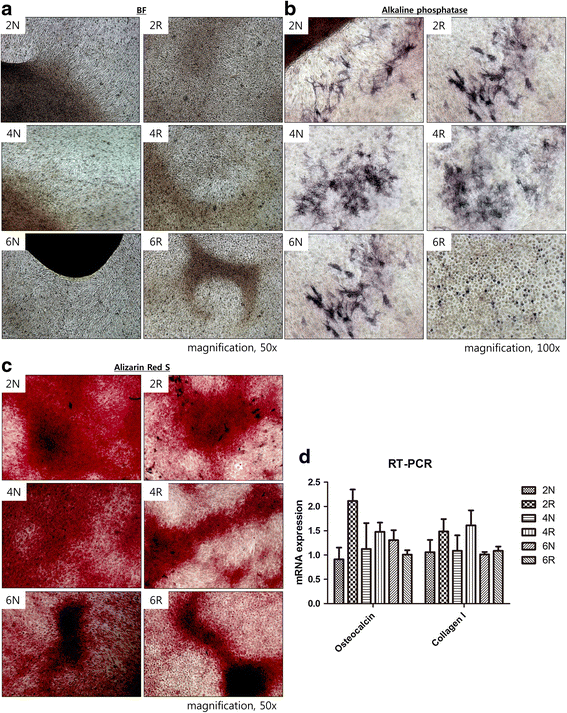
Results: Irradiated ASCs demonstrated significantly decreased proliferative capacity 6 weeks after irradiation. As well, the cells underwent senescence, which was confirmed by blunted morphology, weak mesenchymal cell surface marker expression, and elevated β-galactosidase activity. Irradiated ASCs also exhibited significant losses in the capacity for adipocyte and chondrocyte differentiation. In contrast, osteogenic differentiation was preserved in irradiated ASCs.
Conclusions: We observed decreased proliferation and senescence of irradiated ASCs compared to non-irradiated ASCs 6 weeks after irradiation. Furthermore, irradiated ASCs demonstrated impaired adipocyte and chondrocyte differentiation but retained their osteogenic differentiation capacity. Our results could shed light on additional pathogenic effects of late irradiation, including subcutaneous fibrosis and calcinosis.
Keywords: Cell differentiation; Cell proliferation; Mesenchymal stromal cells; Radiation; Senescence; Swine.
Figures

Similar articles
-
Adipose mesenchymal stromal cells response to ionizing radiation.Cytotherapy. 2016 Mar;18(3):384-401. doi: 10.1016/j.jcyt.2015.12.001. Epub 2016 Jan 15. Cytotherapy. 2016. PMID: 26780866
-
Centrifugal gravity-induced BMP4 induces chondrogenic differentiation of adipose-derived stem cells via SOX9 upregulation.Stem Cell Res Ther. 2016 Dec 8;7(1):184. doi: 10.1186/s13287-016-0445-6. Stem Cell Res Ther. 2016. PMID: 27931264 Free PMC article.
-
Effects of Cryopreservation on Canine Multipotent Stromal Cells from Subcutaneous and Infrapatellar Adipose Tissue.Stem Cell Rev Rep. 2016 Apr;12(2):257-68. doi: 10.1007/s12015-015-9634-4. Stem Cell Rev Rep. 2016. PMID: 26537238 Free PMC article.
-
Function of microRNAs in the Osteogenic Differentiation and Therapeutic Application of Adipose-Derived Stem Cells (ASCs).Int J Mol Sci. 2017 Dec 2;18(12):2597. doi: 10.3390/ijms18122597. Int J Mol Sci. 2017. PMID: 29207475 Free PMC article. Review.
-
Adipose Stromal Cell Expansion and Exhaustion: Mechanisms and Consequences.Cells. 2020 Apr 2;9(4):863. doi: 10.3390/cells9040863. Cells. 2020. PMID: 32252348 Free PMC article. Review.
Cited by
-
Angiogenic CD34+CD146+ adipose-derived stromal cells augment recovery of soft tissue after radiotherapy.J Tissue Eng Regen Med. 2021 Dec;15(12):1105-1117. doi: 10.1002/term.3253. Epub 2021 Oct 5. J Tissue Eng Regen Med. 2021. PMID: 34582109 Free PMC article.
-
Sex-based differences in the severity of radiation-induced arthrofibrosis.J Orthop Res. 2022 Nov;40(11):2586-2596. doi: 10.1002/jor.25297. Epub 2022 Feb 21. J Orthop Res. 2022. PMID: 35148568 Free PMC article.
-
An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity.Adv Sci (Weinh). 2023 Jul;10(20):e2207334. doi: 10.1002/advs.202207334. Epub 2023 May 10. Adv Sci (Weinh). 2023. PMID: 37162248 Free PMC article. Review.
-
Impact of X-ray Exposure on the Proliferation and Differentiation of Human Pre-Adipocytes.Int J Mol Sci. 2018 Sep 11;19(9):2717. doi: 10.3390/ijms19092717. Int J Mol Sci. 2018. PMID: 30208657 Free PMC article.
-
Stromal Vascular Fraction Cells from Individuals Who Have Previously Undergone Radiotherapy Retain Their Pro-Wound Healing Properties.J Clin Med. 2023 Mar 4;12(5):2052. doi: 10.3390/jcm12052052. J Clin Med. 2023. PMID: 36902839 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

