Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries
- PMID: 27530399
- PMCID: PMC4986836
- DOI: 10.1136/bmj.i3903
Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries
Abstract
Objective: To evaluate the association between pioglitazone use and bladder cancer risk in patients with type 2 diabetes.
Design: Retrospective cohort study using propensity score matched cohorts.
Settings: Healthcare databases from Finland, the Netherlands, Sweden, and the United Kingdom. Data comprised country specific datasets of linked records on prescriptions, hospitals, general practitioners, cancer, and deaths.
Participants: Patients with type 2 diabetes who initiated pioglitazone (n=56 337) matched with patients with type 2 diabetes in the same country exposed to diabetes drug treatments other than pioglitazone (n=317 109). Two matched cohorts were created, using a 1:1 fixed ratio (nearest match cohort) and a 1:10 variable ratio (multiple match cohort). Patients were matched on treatment history and propensity scores accounting for several variables associated with pioglitazone initiation.
Main outcome measures: Hazard ratios and 95% confidence intervals were estimated by Cox's proportional hazards model with adjustments for relevant confounders. To assess the robustness of the findings, several sensitivity and stratified analyses were performed.
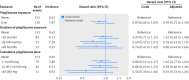
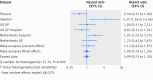
Results: In the cohort exposed to pioglitazone treatment, 130 bladder cancers occurred over a mean follow-up time of 2.9 years. In the nearest match and multiple match cohorts not exposed to pioglitazone treatment, 153 and 970 bladder cancers were recorded, with a mean follow‑up time of 2.8 and 2.9 years, respectively. With regards to bladder cancer risk, the adjusted hazard ratio for patients ever exposed versus never exposed to pioglitazone was 0.99 (95% confidence interval 0.75 to 1.30) and 1.00 (0.83 to 1.21) in the nearest and multiple match cohorts, respectively. Increasing duration of pioglitazone use and increasing cumulative dose were not associated with risk of bladder cancer (>48 months of pioglitazone use, adjusted hazard ratio 0.86 (0.44 to 1.66); >40 000 mg cumulative dose, 0.65 (0.33 to 1.26) in the nearest match cohort).
Conclusions: This study shows no evidence of an association between ever use of pioglitzone and risk of bladder cancer compared with never use, which is consistent with results from other recent studies that also included a long follow-up period.
Trial registration: Registered to the European Union electronic register of post-authorisation studies (EU PAS register no EUPAS3626).
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures



References
-
- Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279-89. 10.1016/S0140-6736(05)67528-9 pmid:16214598. - DOI - PubMed
-
- Ryder RE, Defronzo RA. Rehabilitation of pioglitazone. Br J Diabetes Vasc Dis 2015;15:46-910.15277/bjdvd.2015.021. - DOI
-
- Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn AR, Perez A. Observational follow-up of the PROactive study: a 6-year update. Diabetes Obes Metab 2014;16:63-74. 10.1111/dom.12180 pmid:23859428. - DOI - PubMed
-
- Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ 2012;184:E675-83. 10.1503/cmaj.112102 pmid:22761478. - DOI - PMC - PubMed
-
- Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist 2013;18:148-56. 10.1634/theoncologist.2012-0302 pmid:23345544. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
