Pathophysiology of the inner ear after blast injury caused by laser-induced shock wave
- PMID: 27531021
- PMCID: PMC4987642
- DOI: 10.1038/srep31754
Pathophysiology of the inner ear after blast injury caused by laser-induced shock wave
Abstract
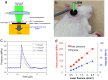
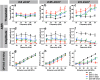
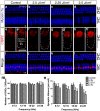
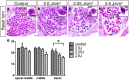
The ear is the organ that is most sensitive to blast overpressure, and ear damage is most frequently seen after blast exposure. Blast overpressure to the ear results in sensorineural hearing loss, which is untreatable and is often associated with a decline in the quality of life. In this study, we used a rat model to demonstrate the pathophysiological and structural changes in the inner ear that replicate pure sensorineural hearing loss associated with blast injury using laser-induced shock wave (LISW) without any conductive hearing loss. Our results indicate that threshold elevation of the auditory brainstem response (ABR) after blast exposure was primarily caused by outer hair cell dysfunction induced by stereociliary bundle disruption. The bundle disruption pattern was unique; disturbed stereocilia were mostly observed in the outermost row, whereas those in the inner and middle rows stereocilia remained intact. In addition, the ABR examination showed a reduction in wave I amplitude without elevation of the threshold in the lower energy exposure group. This phenomenon was caused by loss of the synaptic ribbon. This type of hearing dysfunction has recently been described as hidden hearing loss caused by cochlear neuropathy, which is associated with tinnitus or hyperacusis.
Figures

References
-
- Gallun F. J. et al.. Implications of blast exposure for central auditory function: a review. J Rehabil Res Dev 49, 1059–1074 (2012). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

