Randomized placebo control study of insulin sensitizers (Metformin and Pioglitazone) in psoriasis patients with metabolic syndrome (Topical Treatment Cohort)
- PMID: 27531132
- PMCID: PMC4987981
- DOI: 10.1186/s12895-016-0049-y
Randomized placebo control study of insulin sensitizers (Metformin and Pioglitazone) in psoriasis patients with metabolic syndrome (Topical Treatment Cohort)
Abstract
Background: Increased prevalence of metabolic syndrome (MS) is observed in psoriasis. Metformin has shown improvement in cardiovascular risk factors while pioglitazone demonstrated anti proliferative, anti-inflammatory and anti angiogenic effects. Study objective is to evaluate the efficacy and safety of Insulin sensitizers (metformin and pioglitazone) in psoriasis patients with metabolic syndrome (MS).
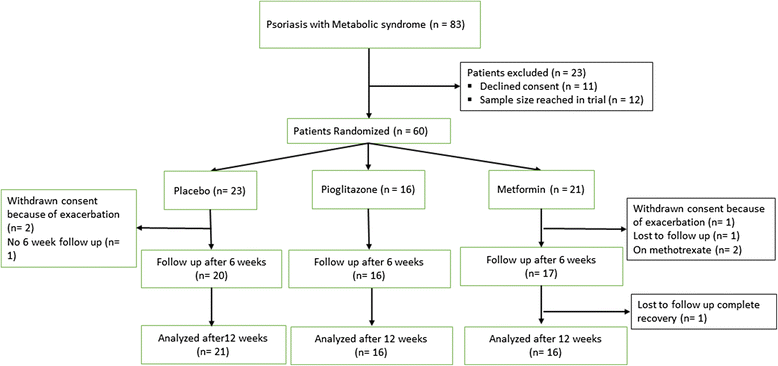
Methods: Single centre, parallel group, randomized, study of metformin, pioglitazone and placebo in psoriasis patients with MS.
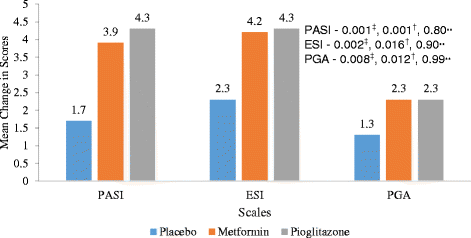
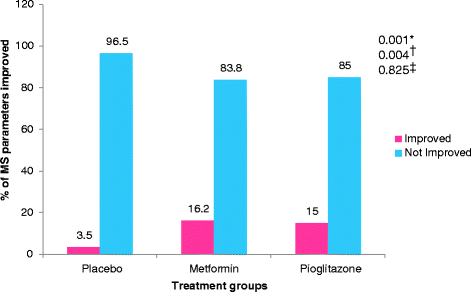
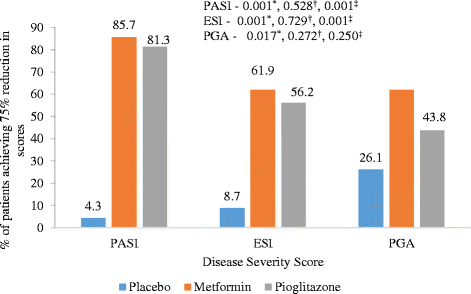
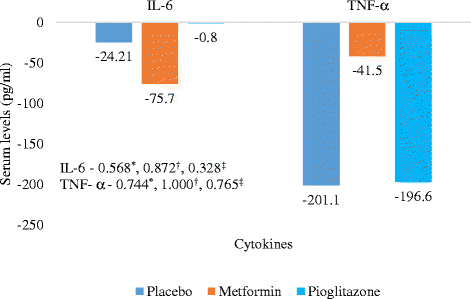
Results: Statistically significant improvement was observed in Psoriasis Area and Severity Index (PASI), Erythema, Scaling and Induration (ESI) and Physician global assessment (PGA) scores in pioglitazone (p values - PASI = 0.001, ESI = 0.002, PGA = 0.008) and metformin groups (p values - PASI = 0.001, ESI = 0.016, PGA = 0.012) as compared to placebo. There was statistically significant difference in percentage of patients achieving 75 % reduction in PASI and ESI scores in metformin (p value - PASI = 0.001, ESI = 0.001) and pioglitazone groups (p vaue - PASI = 0.001, ESI = 0.001). Significant improvement was observed in fasting plasma glucose (FPG) and triglycerides levels in metformin and pioglitazone arms. Significant improvement was noted in weight, BMI, waist circumference, FPG, triglycerides and total cholesterol after 12 weeks of treatment with metformin while pioglitazone showed improvement in FPG, triglyceride levels, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol and LDL cholesterol levels. There was no difference in pattern of adverse drug reaction in three groups.
Conclusion: Insulin sensitizers have shown improvement in the parameters of MS as well as disease severity in psoriasis patients.
Trial registration: CTRI Registration Number: CTRI/2011/12/002252 . Registered on 19/12/2011.
Keywords: Insulin sensitizers; Metabolic syndrome; Metformin; Pioglitazone; Psoriasis.
Figures
Similar articles
-
Randomized Placebo Control Study of Metformin in Psoriasis Patients with Metabolic Syndrome (Systemic Treatment Cohort).Indian J Endocrinol Metab. 2017 Jul-Aug;21(4):581-587. doi: 10.4103/ijem.IJEM_46_17. Indian J Endocrinol Metab. 2017. PMID: 28670544 Free PMC article.
-
Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial.J Clin Endocrinol Metab. 2004 Dec;89(12):6068-76. doi: 10.1210/jc.2003-030861. J Clin Endocrinol Metab. 2004. PMID: 15579760 Clinical Trial.
-
Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients.Metabolism. 2010 Jun;59(6):887-95. doi: 10.1016/j.metabol.2009.10.007. Epub 2009 Dec 16. Metabolism. 2010. PMID: 20015525 Clinical Trial.
-
The efficacy of metformin for the treatment of psoriasis: a meta-analysis study.Postepy Dermatol Alergol. 2023 Oct;40(5):606-610. doi: 10.5114/ada.2023.130524. Epub 2023 Aug 22. Postepy Dermatol Alergol. 2023. PMID: 38028405 Free PMC article. Review.
-
[Pioglitazone effects on blood pressure in patients with metabolic syndrome].Nihon Rinsho. 2008 Aug;66(8):1591-5. Nihon Rinsho. 2008. PMID: 18700562 Review. Japanese.
Cited by
-
Psoriasis and Cardiovascular Comorbidities: Focusing on Severe Vascular Events, Cardiovascular Risk Factors and Implications for Treatment.Int J Mol Sci. 2017 Oct 21;18(10):2211. doi: 10.3390/ijms18102211. Int J Mol Sci. 2017. PMID: 29065479 Free PMC article. Review.
-
A Molecular Perspective on the Potential Benefits of Metformin for the Treatment of Inflammatory Skin Disorders.Int J Mol Sci. 2020 Nov 25;21(23):8960. doi: 10.3390/ijms21238960. Int J Mol Sci. 2020. PMID: 33255783 Free PMC article. Review.
-
Metformin Promotes HaCaT Cell Apoptosis through Generation of Reactive Oxygen Species via Raf-1-ERK1/2-Nrf2 Inactivation.Inflammation. 2018 Jun;41(3):948-958. doi: 10.1007/s10753-018-0749-z. Inflammation. 2018. PMID: 29549478
-
Effects of metformin on blood lipid profiles in nondiabetic adults: a meta-analysis of randomized controlled trials.Endocrine. 2020 Feb;67(2):305-317. doi: 10.1007/s12020-020-02190-y. Epub 2020 Jan 16. Endocrine. 2020. PMID: 31950354
-
Efficacy and safety of various drug combinations in treating plaque Psoriasis: A meta-analysis.F1000Res. 2025 Jan 28;13:453. doi: 10.12688/f1000research.149172.2. eCollection 2024. F1000Res. 2025. PMID: 39925996 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

