Selective Gas-Phase Ion/Ion Reactions: Enabling Disulfide Mapping via Oxidation and Cleavage of Disulfide Bonds in Intermolecularly-Linked Polypeptide Ions
- PMID: 27531151
- PMCID: PMC5068824
- DOI: 10.1021/acs.analchem.6b01043
Selective Gas-Phase Ion/Ion Reactions: Enabling Disulfide Mapping via Oxidation and Cleavage of Disulfide Bonds in Intermolecularly-Linked Polypeptide Ions
Abstract
The selective gas-phase oxidation of disulfide bonds to their thiosulfinate form using ion/ion reactions and subsequent cleavage is demonstrated here. Oxidizing reagent anions are observed to attach to all polypeptides, regardless of amino acid composition. Direct proton transfer yielding a charge-reduced peptide is also frequently observed. Activation of the ion/ion complex between an oxidizing reagent anion and a disulfide-containing peptide cation results in oxygen transfer from the reagent anion to the peptide cation to form the [M+H+O](+) species. This thiosulfinate derivative can undergo one of several rearrangements that result in cleavage of the disulfide bond. Species containing an intermolecular disulfide bond undergo separation of the two chains upon activation. Further activation can be used to generate more sequence information from each chain. These oxidation ion/ion reactions have been used to illustrate the identification of S-glutathionylated and S-cysteinylated peptides, in which low molecular weight thiols are attached to cysteine residues in peptides via disulfide bonds. The oxidation chemistry effectively labels peptide ions with readily oxidized groups, such as disulfide bonds. This enables a screening approach for the identification of disulfide-linked peptides in a disulfide mapping application involving enzymatic digestion. The mixtures of ions generated by tryptic and peptic digestions of lysozyme and insulin, respectively, without prior separation or isolation were subjected both to oxidation and proton transfer ion/ion chemistry to illustrate the identification of peptides in the mixtures with readily oxidized groups.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



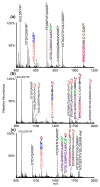
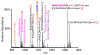

Similar articles
-
Selective Gas-Phase Oxidation and Localization of Alkylated Cysteine Residues in Polypeptide Ions via Ion/Ion Chemistry.J Proteome Res. 2016 Sep 2;15(9):3139-46. doi: 10.1021/acs.jproteome.6b00266. Epub 2016 Aug 18. J Proteome Res. 2016. PMID: 27476698 Free PMC article.
-
Ion trap collisional activation of disulfide linkage intact and reduced multiply protonated polypeptides.Rapid Commun Mass Spectrom. 1999;13(20):2040-8. doi: 10.1002/(SICI)1097-0231(19991030)13:20<2040::AID-RCM754>3.0.CO;2-W. Rapid Commun Mass Spectrom. 1999. PMID: 10510418
-
Oxidation of methionine residues in polypeptide ions via gas-phase ion/ion chemistry.J Am Soc Mass Spectrom. 2014 Jun;25(6):1049-57. doi: 10.1007/s13361-014-0861-8. Epub 2014 Mar 27. J Am Soc Mass Spectrom. 2014. PMID: 24671696 Free PMC article.
-
New disulfide bond-forming reactions for peptide and protein synthesis.Braz J Med Biol Res. 1994 Dec;27(12):2733-44. doi: 10.1002/chin.199620250. Braz J Med Biol Res. 1994. PMID: 7549997 Review.
-
[A novel synthetic approach of tryptophan-containing cystine peptides by regioselective disulfide bond-forming reaction using the silyl chloride-sulfoxide system].Yakugaku Zasshi. 2000 Feb;120(2):197-205. doi: 10.1248/yakushi1947.120.2_197. Yakugaku Zasshi. 2000. PMID: 10689966 Review. Japanese.
Cited by
-
The Role of Electron Transfer Dissociation in Modern Proteomics.Anal Chem. 2018 Jan 2;90(1):40-64. doi: 10.1021/acs.analchem.7b04810. Epub 2017 Dec 12. Anal Chem. 2018. PMID: 29172454 Free PMC article. Review. No abstract available.
-
Gas-Phase Oxidation of Neutral Basic Residues in Polypeptide Cations by Periodate.J Am Soc Mass Spectrom. 2016 Dec;27(12):1979-1988. doi: 10.1007/s13361-016-1491-0. Epub 2016 Sep 19. J Am Soc Mass Spectrom. 2016. PMID: 27644939 Free PMC article.
-
Top-Down Analysis of Disulfide-Linked Proteins Using Photoinduced Radical Reactions and ET-DDC.Int J Mass Spectrom. 2019 Oct;444:116173. doi: 10.1016/j.ijms.2019.06.009. Epub 2019 Jul 2. Int J Mass Spectrom. 2019. PMID: 31372092 Free PMC article.
-
Design of a TW-SLIM Module for Dual Polarity Confinement, Transport, and Reactions.J Am Soc Mass Spectrom. 2017 Jul;28(7):1442-1449. doi: 10.1007/s13361-017-1680-5. Epub 2017 May 30. J Am Soc Mass Spectrom. 2017. PMID: 28560562 Free PMC article.
-
Recent Developments in Gas-Phase Ion/Ion Reactions for Analytical Mass Spectrometry.Anal Chem. 2020 Jan 7;92(1):252-266. doi: 10.1021/acs.analchem.9b05014. Epub 2019 Nov 26. Anal Chem. 2020. PMID: 31693342 Free PMC article. Review.
References
-
- Pantoliano MW, Ladner RC, Bryan PN, Rollence ML, Wood JF, Poulos TL. Biochemistry. 1987;26:2077–2082. - PubMed
-
- Cook KM, Hogg PJ. Antioxid Redox Signaling. 2013;18:1987–2015. - PubMed
-
- Leonard SE, Carroll KS. Curr Opin Chem Biol. 2011;15:88–102. - PubMed
-
- Reynaert NL, Ckless K, Guala AS, Wouters EFM, van der Vliet A, Janssen-Heininger YMW. Biochim Biophys Acta, Gen Subj. 2006;1760:380–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

