Treatment of Lassa virus infection in outbred guinea pigs with first-in-class human monoclonal antibodies
- PMID: 27531367
- PMCID: PMC5032844
- DOI: 10.1016/j.antiviral.2016.08.012
Treatment of Lassa virus infection in outbred guinea pigs with first-in-class human monoclonal antibodies
Abstract
Lassa fever is a significant health threat to West African human populations with hundreds of thousands of annual cases. There are no approved medical countermeasures currently available. Compassionate use of the antiviral drug ribavirin or transfusion of convalescent serum has resulted in mixed success depending on when administered or the donor source, respectively. We previously identified several recombinant human monoclonal antibodies targeting the glycoprotein of Lassa virus with strong neutralization profiles in vitro. Here, we demonstrate remarkable therapeutic efficacy using first-in-class human antibodies in a guinea pig model of Lassa infection thereby presenting a promising treatment alternative.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
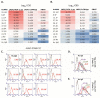
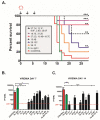
 ) were treated with LASV huMAbs, at 30 mg/kg of body weight on the same day (↓). Additional therapeutic antibody was administered at the same dose on days 3 and 6 PI (↓). Animals were monitored for clinical signs of LF throughout the 28-day study timeline. (B.) Viremia data from treated and control GP plasma on days 7 and 14 PI. Viremia levels for day 7 treatment groups 37.7H, 12.1F, and 25.6A as well as day 14 12.1F, 37.2D, 19.7E, and 10.4B were below the limit of detection (LOD). Error bars represent standard deviation from mean values. * denotes P≤0.05. ** denotes P≤0.001. *** denotes P≤0.0001.
) were treated with LASV huMAbs, at 30 mg/kg of body weight on the same day (↓). Additional therapeutic antibody was administered at the same dose on days 3 and 6 PI (↓). Animals were monitored for clinical signs of LF throughout the 28-day study timeline. (B.) Viremia data from treated and control GP plasma on days 7 and 14 PI. Viremia levels for day 7 treatment groups 37.7H, 12.1F, and 25.6A as well as day 14 12.1F, 37.2D, 19.7E, and 10.4B were below the limit of detection (LOD). Error bars represent standard deviation from mean values. * denotes P≤0.05. ** denotes P≤0.001. *** denotes P≤0.0001.References
-
- Haas WH, Breuer T, Pfaff G, Schmitz H, Kohler P, Asper M, Emmerich P, Drosten C, Golnitz U, Fleischer K, Gunther S. Imported Lassa fever in Germany: surveillance and management of contact persons. Clin. Infect. Dis. 2003;36:1254–1258. - PubMed

