Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib
- PMID: 27531525
- PMCID: PMC5303616
- DOI: 10.1002/ajh.24536
Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib
Abstract
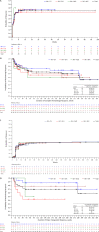
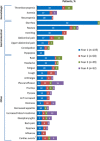
Bosutinib is an Src/Abl tyrosine kinase inhibitor (TKI) indicated for adults with Ph+ chronic myeloid leukemia (CML) resistant/intolerant to prior TKIs. This long-term update of an ongoing phase 1/2 study evaluated the efficacy and safety of third-/fourth-line bosutinib in adults with chronic phase (CP) CML. Median durations of treatment and follow-up were 8.6 (range, 0.2-87.7) months and 32.7 (0.3-93.3) months, respectively. Cumulative confirmed complete hematologic response (cCHR) and major cytogenetic response (MCyR) rates were 74% (95% CI, 65-81%) and 40% (31-50%), respectively; Kaplan-Meier (K-M) probability of maintaining cCHR or MCyR at 4 years was 63% (95% CI, 50-73%) and 69% (52-81%). Cumulative incidence of on-treatment disease progression (PD)/death at 4 years was 24% (95% CI, 17-33%); K-M 4-year overall survival was 78% (68-85%). Baseline Ph+ cells ≤35 vs. ≥95% was prognostic of MCyR and CCyR by 3 and 6 months, increased baseline basophils was prognostic of PD/death, and no prior response to second-line TKI was prognostic of death. Common adverse events included diarrhea (83%), nausea (48%), vomiting (38%), and thrombocytopenia (39%). Bosutinib demonstrates durable efficacy and a toxicity profile similar to previous bosutinib studies in CP CML patients resistant/intolerant to multiple TKIs, representing an important treatment option for patients in this setting. This trial is registered at www.clinicaltrials.gov (NCT00261846). Am. J. Hematol. 91:1206-1214, 2016. © 2016 Wiley Periodicals, Inc.
© 2016 Wiley Periodicals, Inc.
Figures
References
-
- Jain P, Kantarjian H, Cortes J. Chronic myeloid leukemia: Overview of new agents and comparative analysis. Curr Treat Options Oncol 2013;14:127–143. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR–ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1031–1037. - PubMed
-
- Hochhaus A, O'Brien SG, Guilhot F, et al. Six‐year follow‐up of patients receiving imatinib for the first‐line treatment of chronic myeloid leukemia. Leukemia 2009; 23:1054–1061. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
