Osmotic and stimulant laxatives for the management of childhood constipation
- PMID: 27531591
- PMCID: PMC6513425
- DOI: 10.1002/14651858.CD009118.pub3
Osmotic and stimulant laxatives for the management of childhood constipation
Abstract
Background: Constipation within childhood is an extremely common problem. Despite the widespread use of osmotic and stimulant laxatives by health professionals to manage constipation in children, there has been a long standing paucity of high quality evidence to support this practice.
Objectives: We set out to evaluate the efficacy and safety of osmotic and stimulant laxatives used to treat functional childhood constipation.
Search methods: We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane IBD Group Specialized Trials Register from inception to 10 March 2016. There were no language restrictions. We also searched the references of all included studies, personal contacts and drug companies to identify studies.
Selection criteria: Randomised controlled trials (RCTs) which compared osmotic or stimulant laxatives to placebo or another intervention, with participants aged 0 to 18 years old were considered for inclusion. The primary outcome was frequency of defecation. Secondary endpoints included faecal incontinence, disimpaction, need for additional therapies and adverse events.
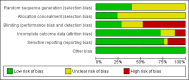
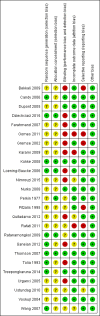
Data collection and analysis: Relevant papers were identified and two authors independently assessed the eligibility of trials, extracted data and assessed methodological quality using the Cochrane risk of bias tool. The primary outcome was frequency of defecation. Secondary endpoints included faecal incontinence, disimpaction, need for additional therapies and adverse events. For continuous outcomes we calculated the mean difference (MD) and 95% confidence interval (CI) using a fixed-effect model. For dichotomous outcomes we calculated the risk ratio (RR) and 95% CI using a fixed-effect model. The Chi(2) and I(2) statistics were used to assess statistical heterogeneity. A random-effects model was used in situations of unexplained heterogeneity. We assessed the overall quality of the evidence supporting the primary and secondary outcomes using the GRADE criteria.
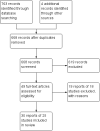
Main results: Twenty-five RCTs (2310 participants) were included in the review. Fourteen studies were judged to be at high risk of bias due to lack of blinding, incomplete outcome data and selective reporting. Meta-analysis of two studies (101 patients) comparing polyethylene glycol (PEG) with placebo showed a significantly increased number of stools per week with PEG (MD 2.61 stools per week, 95% CI 1.15 to 4.08). Common adverse events in the placebo-controlled studies included flatulence, abdominal pain, nausea, diarrhoea and headache. Participants receiving high dose PEG (0.7 g/kg) had significantly more stools per week than low dose PEG (0.3 g/kg) participants (1 study, 90 participants, MD 1.30, 95% 0.76 to 1.84). Meta-analysis of 6 studies with 465 participants comparing PEG with lactulose showed a significantly greater number of stools per week with PEG (MD 0.70 , 95% CI 0.10 to 1.31), although follow-up was short. Patients who received PEG were significantly less likely to require additional laxative therapies. Eighteen per cent (27/154) of PEG patients required additional therapies compared to 31% (47/150) of lactulose patients (RR 0.55, 95% CI 0.36 to 0.83). No serious adverse events were reported with either agent. Common adverse events in these studies included diarrhoea, abdominal pain, nausea, vomiting and pruritis ani. Meta-analysis of 3 studies with 211 participants comparing PEG with milk of magnesia showed that the stools per week were significantly greater with PEG (MD 0.69, 95% CI 0.48 to 0.89). However, the magnitude of this difference was quite small and may not be clinically significant. One child was noted to be allergic to PEG, but there were no other serious adverse events reported. One study found a significant difference in stools per week favouring milk of magnesia over lactulose (MD -1.51, 95% CI -2.63 to -0.39, 50 patients), Meta-analysis of 2 studies with 287 patients comparing liquid paraffin (mineral oil) with lactulose revealed a relatively large statistically significant difference in the number of stools per week favouring liquid paraffin (MD 4.94 , 95% CI 4.28 to 5.61). No serious adverse events were reported. Adverse events included abdominal pain, distention and watery stools. No statistically significant differences in the number of stools per week were found between PEG and enemas (1 study, 90 patients, MD 1.00, 95% CI -1.58 to 3.58), dietary fibre mix and lactulose (1 study, 125 patients, P = 0.481), senna and lactulose (1 study, 21 patients, P > 0.05), lactitol and lactulose (1 study, 51 patients, MD -0.80, 95% CI -2.63 to 1.03), hydrolyzed guar gum and lactulose (1 study, 61 patients, MD 1.00, 95% CI -1.80 to 3.80), PEG and flixweed (1 study, 109 patients, MD 0.00, 95% CI -0.33 to 0.33), PEG and dietary fibre (1 study, 83 patients, MD 0.20, 95% CI -0.64 to 1.04), and PEG and liquid paraffin (2 studies, 261 patients, MD 0.35, 95% CI -0.24 to 0.95).
Authors' conclusions: The pooled analyses suggest that PEG preparations may be superior to placebo, lactulose and milk of magnesia for childhood constipation. GRADE analyses indicated that the overall quality of the evidence for the primary outcome (number of stools per week) was low or very low due to sparse data, inconsistency (heterogeneity), and high risk of bias in the studies in the pooled analyses. Thus, the results of the pooled analyses should be interpreted with caution because of quality and methodological concerns, as well as clinical heterogeneity, and short follow-up. There is also evidence suggesting the efficacy of liquid paraffin (mineral oil). There is no evidence to demonstrate the superiority of lactulose when compared to the other agents studied, although there is a lack of placebo controlled studies. Further research is needed to investigate the long term use of PEG for childhood constipation, as well as the role of liquid paraffin. The optimal dose of PEG also warrants further investigation.
Conflict of interest statement
Morris Gordon has received travel expenses from Tillotts, Ferring, Clinova, and Danone to attend scientific meetings and present results or chair sessions. All of these financial activities are outside the scope of the present review.
John K MacDonald: None known.
Claire E Parker: None known.
Anthony K Akobeng: None known.
Adrian G Thomas: None known.
Figures
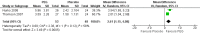































Update of
-
Osmotic and stimulant laxatives for the management of childhood constipation.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD009118. doi: 10.1002/14651858.CD009118.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Aug 17;(8):CD009118. doi: 10.1002/14651858.CD009118.pub3. PMID: 22786523 Updated.
References
References to studies included in this review
Bekkali 2009 {published data only}
-
- Bekkali NL, Dijkgraaf MG, Berg MM, Wijk MP, Bongers ME, Liem O, et al. Treatment of rectal fecal impaction a randomized controlled trial: Rectal enemas versus high doses of oral PEG 3350. Gastroenterology 2009;136(5 Suppl 1):A128‐9.
-
- Bekkali NL, Berg MM, Dijkgraaf MG, Wijk MP, Bongers ME, Liem O, et al. Rectal fecal impaction treatment in childhood constipation: enemas versus high doses oral PEG. Pediatrics 2009;124(6):e1108‐15. - PubMed
Candy 2006 {published data only}
-
- Candy DC, Edwards D, Geraint M. Treatment of faecal impaction with polyethylene glycol plus electrolytes (PGE + E) followed by a double‐blind comparison of PEG + E versus lactulose as maintenance therapy. Journal of Pediatric Gastroenterology and Nutrition 2006;43(1):65‐70. - PubMed
Dupont 2005 {published data only}
-
- Dupont C, Leluyer B, Maamri N, Morali A, Joye J, Fiorini J, et al. Double‐blind randomized evaluation of clinical and biological tolerance of polyethylene glycol 4000 versus lactulose in constipated children. Journal of Pediatric Gastroenterology and Nutrition 2005;41(5):625‐33. - PubMed
Dziechciarz 2015 {published data only}
-
- Dziechciarz P, Horvath A, Szajewska H. Polyethylene glycol 4000 for treatment of functional constipation in children. Journal of Pediatric Gastroenterology and Nutrition 2015;60(1):65‐8. - PubMed
Farahmand 2007 {published data only}
-
- Farahmand F. A randomised trial of liquid paraffin versus lactulose in the treatment of chronic functional constipation in children. Acta Medica Iranica 2007;45:183‐8.
Gomes 2011 {published data only}
-
- Gomes PB, Duarte MA, Melo Mdo C. Comparison of the effectiveness of polyethylene glycol 4000 without electrolytes and magnesium hydroxide in the treatment of chronic functional constipation in children. Jornal de Pediatria 2011;87(1):24‐8. - PubMed
Gremse 2002 {published data only}
-
- Gremse DA, Hixon J, Crutchfield A. Comparison of polyethylene glycol 3350 and lactulose for treatment of chronic constipation in children. Clinical Pediatrics 2002;41(4):225‐9. - PubMed
Karami 2009 {published data only}
-
- Karami H, Khademloo M, Niari P. Polyethylene glycol versus paraffin for the treatment of childhood functional constipation. Iranian Journal of Pediatrics 2009;19(3):255–61.
Kokke 2008 {published data only}
-
- Kokke FT, Scholtens PA, Alles MS, Decates TS, Fiselier TJ, Tolboom JJ, et al. A dietary fiber mixture versus lactulose in the treatment of childhood constipation: a double‐blind randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2008;47(5):592‐7. - PubMed
Loening‐Baucke 2006 {published data only}
-
- Loening‐Baucke V, Pashankar D. A randomized, prospective, comparison study of polyethylene glycol 3350 without electrolytes and milk of magnesia for children with constipation and fecal incontinence. Pediatrics 2006;118(2):528‐35. - PubMed
Nimrouzi 2015 {published data only}
Nurko 2008 {published and unpublished data}
-
- Nurko S, Youssef NN, Sabri M, Langseder A, McGowan J, Cleveland M, et al. PEG3350 in the treatment of childhood constipation: a multicenter, double‐blinded, placebo‐controlled trial. Journal of Pediatrics 2008;153(2):254‐61. - PubMed
Perkin 1977 {published data only}
-
- Perkin JM. Constipation in childhood: a controlled comparison between lactulose and standardized senna. Current Medical Research and Opinion 1977;4(8):540‐3. - PubMed
Pitzalis 1995 {published data only}
-
- Pitzalis G, Deganello F, Mariani P, Chiarini‐Testa MB, Virgilii F, Gasparri R, Calvani L, Bonamico M. Lactitol in chronic idiopathic constipation in children. Pediatria Medica e Chirurgica 1995;17(3):223‐6. - PubMed
Quitadamo 2012 {published data only}
-
- Quitadamo P, Coccorullo P, Giudice E, Mallardo S, Ferra V, Poli E, et al. Prospective, randomized, controlled, multicenter study on the effectiveness of polyethylene glycol 3350 with electrolytes versus a mixture of acacia fiber, psyllium fiber and fructose in the treatment of chronic functional constipation in childhood. Digestive and Liver Disease 2010;42:S355. - PubMed
-
- Quitadamo P, Coccorullo P, Giannetti E, Romano C, Chiaro A, Campanozzi A, et al. A randomized, prospective, comparison study of a mixture of acacia fiber, psyllium fiber, and fructose vs polyethylene glycol 3350 with electrolytes for the treatment of chronic functional constipation in childhood. Journal of Pediatrics 2012;161(4):710‐5. - PubMed
Rafati 2011 {published data only}
Ratanamongkol 2009 {published data only}
-
- Ratanamongkola P, Lertmaharitb S, Jongpiputvanichc S. Polyethylene glycol 4000 without electrolytes versus milk of magnesia for the treatment of functional constipation in infants and young children: A randomized controlled trial. Asian Biomedicine 2009;3(4):391‐9.
Saneian 2012 {published data only}
-
- Saneian H, Mostofizadeh N. Comparing the efficacy of polyethylene glycol (PEG), magnesium hydroxide and lactulosein treatment of functional constipation in children. Journal of Research in Medical Sciences 2012;17(1 Suppl 1):S145‐9.
Thomson 2007 {published and unpublished data}
Tolia 1993 {published data only}
-
- Tolia V, Lin CH, Elitsur Y. A prospective randomized study with mineral oil and oral lavage solution for treatment of faecal impaction in children. Alimentary Pharmacology and Therapeutics 1993;7(5):523‐9. - PubMed
Treepongkaruna 2014 {published data only}
-
- Treepongkaruna S, Simakachorn N, Pienvichit P, Magis A, Garnier P, Maisonobe P, et al. Efficacy of a polyethylene glycol laxative (PEG, Macrogol 4000) versus lactulose for the treatment of chronic constipation in children. Results of a randomized, double‐blind controlled study performed in Thailand. Gastroenterology 2013;144(5 Suppl 1):S547.
Urganci 2005 {published data only}
-
- Urganci N, Akyildiz B, Polat TB. A comparative study: the efficacy of liquid paraffin and lactulose in management of chronic functional constipation. Pediatrics International 2005;47(1):15‐9. - PubMed
Ustundag 2010 {published data only}
-
- Ustundag G, Kuloglu Z, Kirbas N, Kansu A. Can partially hydrolyzed guar gum be an alternative to lactulose in treatment of childhood constipation?. Turkish Journal of Gastroenterology 2010;21(4):360‐4. - PubMed
-
- Ustundag GH, Kuloglu Z, Kyrbas N, Kansu A. Can partially hydrolyzed guar gum be an alternative to lactulose in treatment of childhood constipation?. Journal of Pediatric Gastroenterology and Nutrition 2010;50:E120. - PubMed
Voskujl 2004 {published data only}
Wang 2007 {published data only}
-
- Wang BX, Wang MG, Jiang MZ, Xu CD, Shao CH, Jia LY, et al. Forlax in the treatment of childhood constipation: a randomized, controlled, multicenter clinical study. Zhongguo Dang Dai Er Ke Za Zhi 2007;9(5):429‐32. - PubMed
References to studies excluded from this review
Bekkali 2011 {published data only}
-
- Bekkali NL, Liem O, Bongers ME, Wijk MP, Pelleboer R, Koot B, et al. One year treatment of childhood constipation comparing PEG 3350 with electrolytes versus PEG 4000: A double blind randomized controlled trial. Gastroenterology 2012;142(5 Suppl 1):S821‐2.
Berg 1983 {published data only}
-
- Berg I, Forsythe I, Holt P, Watts J. A controlled trial of 'Senokot' in faecal soiling treated by behavioural methods. Journal of Child Psychology and Psychiatry and Allied Disciplines 1983;24(4):543‐9. - PubMed
Bongers 2009 {published data only}
-
- Bongers ME, Berg MM, Reitsma JB, Voskuijl WP, Benninga MA. A randomized controlled trial of enemas in combination with oral laxative therapy for children with chronic constipation. Clinical Gastroenterology and Hepatology 2009;7(10):1069‐74. - PubMed
Connolly 1974 {published data only}
-
- Connolly P, Hughes IW, Ryan G. Comparison of "Duphalac" and "irritant" laxatives during and after treatment of chronic constipation: a preliminary study. Current Medical Research and Opinion 1974;2(10):620‐5. - PubMed
Corazziari 1996 {published data only}
-
- Corazziari E, Badiali D, Habib FI, Reboa G, Pitto G, Mazzacca G, et al. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF‐100) in treatment of chronic nonorganic constipation. Digestive Diseases and Sciences 1996;41(8):1636‐40. - PubMed
Dehghani 2014 {published data only}
-
- Dehghani SM, Askarian M, Kaffashan HA. Oral domperidone has no additional effect on chronic functional constipation in children: a randomized clinical trial. Indian Journal of Gastroenterology 2014;33(2):125‐30. - PubMed
Dupont 2006 {published data only}
-
- Dupont C, Leluyer B, Amar F, Kalach N, Benhamou PH, Mouterde O, et al. A dose determination study of polyethylene glycol 4000 in constipated children: factors influencing the maintenance dose. Journal of Pediatric Gastroenterology and Nutrition 2006;42(2):178‐85. - PubMed
Ferguson 1999 {published data only}
-
- Ferguson A, Culbert P, Gillett H, Barras N. New polyethylene glycol electrolyte solution for the treatment of constipation and faecal impaction. Italian Journal of Gastroenterology and Hepatology 1999;31 Supp 3:S249‐52. - PubMed
Hardikar 2007 {published data only}
-
- Hardikar W, Cranswick N, Heine RG. Macrogol 3350 plus electrolytes for chronic constipation in children: a single‐centre, open‐label study. Journal of Paediatrics and Child Health 2007;43(7‐8):527‐31. - PubMed
Hejl 1990 {published data only}
-
- Hejl M, Kamper J, Ebbesen F, Hansted C. Infantile constipation and Allomin‐lactulose. Treatment of infantile obstipation in infants fed with breast milk substitutes. A controlled clinical trial of 2 per cent and 4 per cent Allomin‐lactulose. Ugeskrift for Laeger 1990;152(25):1819‐22. - PubMed
Kazak 1999 {published data only}
-
- Kazak SS, Beketova HV, Voronina SS, Amanbaieva HT. Forlax in the treatment of the constipation syndrome in children with combined digestive organ pathology. Likarska Sprava 1999, (7‐8):142‐7. - PubMed
Khoshoo 2006 {published data only}
-
- Khoshoo V, Armstead C, Landry L. Effect of a laxative with and without tegaserod in adolescents with constipation predominant irritable bowel syndrome. Alimentary Pharmacology and Therapeutics 2006;23(1):191‐6. - PubMed
Miller 2012 {published data only}
-
- Miller MK, Dowd MD, Friesen CA, Walsh‐Kelly CM. A randomized trial of enema versus polyethylene glycol 3350 for fecal disimpaction in children presenting to an emergency department. Pediatric Emergency Care 2012;28(2):115‐9. - PubMed
Ormarsson 2013 {published data only}
-
- Ormarsson OT, Asgrimsdottir GM, Stefansson E, Loftsson T, Bjornsson E. Marine lipid suppositories for constipation in children. Gastroenterology 2013;144(5 Suppl 1):S398‐9.
Savino 2012 {published data only}
Shevtsov 2005 {published data only}
-
- Shevtsov SA. Clinical efficacy of duphalac in the treatment of functional constipations. Ėksperimental'nai͡a i Klinicheskai͡a Gastroėnterologii͡a 2005, (6):58‐60. - PubMed
Sonheimer 1982 {published data only}
-
- Sondheimer JM, Gervaise EP. Lubricant versus laxative in the treatment of chronic functional constipation of children: a comparative study. Journal of Pediatric Gastroenterology and Nutrition 1982;1(2):223‐6. - PubMed
Youssef 2002 {published data only}
-
- Youssef NN, Peters JM, Henderson W, Shultz‐Peters S, Lockhart DK, Lorenzo C. Dose response of PEG 3350 for the treatment of childhood fecal impaction. Journal of Pediatrics 2002;141(3):410‐4. - PubMed
Additional references
Anonymous 2006
-
- Anonymous. Evaluation and treatment of constipation in children: summary of updated recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2006;43(3):405‐7. - PubMed
Anonymous 2010
-
- Anonymous. Constipation in children and young people: diagnosis and management of idiopathic childhood constipation in primary and secondary care. Available from http://www.nice.org.uk/nicemedia/live/12993/48721/48721.pdf [Accessed 13th July 2010]. Published by the RCOG Press at the Royal College of Obstetricians and Gynaecologists, London, UK, 2010. - PubMed
Baker 1999
-
- Baker SS, Liptak GS, Colletti RB, Croffie JM, Lorenzo C, Ector W, et al. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 1999;29(5):612‐26. - PubMed
Guyatt 2008
Higgins 2003
Higgins 2011a
-
- Hggins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hozo 2005
Hyman 2006
-
- Hyman PE, Milla PJ, Marc A. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 2006;130(5):1519‐26. - PubMed
Lee‐Robichaud 2010
Partin 1992
-
- Partin JC, Hamill SK, Fischel JE, Partin JS. Painful defecation and fecal soiling in children. Pediatrics 1992;89(6 Pt 1):1007‐9. - PubMed
Pijpers 2008
-
- Pijpers MA, Tabbers MM, Benninga MA, Berger MY. Currently recommended treatments of childhood constipation are not evidence based: a systematic literature review on the effect of laxative treatment and dietary measures. Archives of Disease in Childhood 2009;94(2):117‐31. - PubMed
Pitkin 1999
-
- Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. JAMA 1999;281(12):1110‐1. - PubMed
Price 2001
Rasquin 2006
Rasquin‐Weber 1999
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Van den Berg 2006
-
- Berg MM, Benninga MA, Lorenzo C. Epidemiology of childhood constipation: a systematic review. American Journal of Gastroenterology 2006;101(10):2401‐9. - PubMed
Zanetti 2007
-
- Zanetti G, Marchiori E, Gasparetto TD, Escuissato DL, Soares Souza A Jr. Lipoid pneumonia in children following aspiration of mineral oil used in the treatment of constipation: high‐resolution CT findings in 17 patients. Pediatric Radiology 2007;37(11):1135‐9. - PubMed
References to other published versions of this review
Gordon 2012
Gordon 2013
-
- Gordon M, Naidoo K, Akobeng AK, Thomas AG. Cochrane Review: Osmotic and stimulant laxatives for the management of childhood constipation (Review). Evidence‐Based Child Health a Cochrane Review Journal 2013;8(1):57‐109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

