The β-amyloid peptide compromises Reelin signaling in Alzheimer's disease
- PMID: 27531658
- PMCID: PMC4987719
- DOI: 10.1038/srep31646
The β-amyloid peptide compromises Reelin signaling in Alzheimer's disease
Abstract
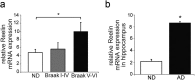
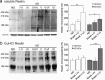
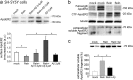
Reelin is a signaling protein that plays a crucial role in synaptic function, which expression is influenced by β-amyloid (Aβ). We show that Reelin and Aβ oligomers co-immunoprecipitated in human brain extracts and were present in the same size-exclusion chromatography fractions. Aβ treatment of cells led to increase expression of Reelin, but secreted Reelin results trapped together with Aβ aggregates. In frontal cortex extracts an increase in Reelin mRNA, and in soluble and insoluble (guanidine-extractable) Reelin protein, was associated with late Braak stages of Alzheimer's disease (AD), while expression of its receptor, ApoER2, did not change. However, Reelin-dependent induction of Dab1 phosphorylation appeared reduced in AD. In cells, Aβ reduced the capacity of Reelin to induce internalization of biotinylated ApoER2 and ApoER2 processing. Soluble proteolytic fragments of ApoER2 generated after Reelin binding can be detected in cerebrospinal fluid (CSF). Quantification of these soluble fragments in CSF could be a tool to evaluate the efficiency of Reelin signaling in the brain. These CSF-ApoER2 fragments correlated with Reelin levels only in control subjects, not in AD, where these fragments diminished. We conclude that while Reelin expression is enhanced in the Alzheimer's brain, the interaction of Reelin with Aβ hinders its biological activity.
Figures




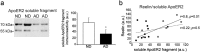
References
-
- D’Arcangelo G. et al. Reelin is a ligand for lipoprotein receptors. Neuron. 24, 471–479 (1999). - PubMed
-
- Hiesberger T. et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuro. 24, 481–489 (1999). - PubMed
-
- May P., Bock H. H., Nimpf J. & Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J Biol Chem. 278, 37386–37392 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

