Immune recognition of citrullinated epitopes
- PMID: 27531825
- PMCID: PMC5011684
- DOI: 10.1111/imm.12640
Immune recognition of citrullinated epitopes
Abstract
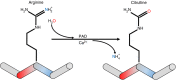
Conversion of arginine into citrulline is a post-translational modification that is observed in normal physiological processes. However, abnormal citrullination can provoke autoimmunity by generating altered self-epitopes that are specifically targeted by autoantibodies and T cells. In this review we discuss the recognition of citrullinated antigens in human autoimmune diseases and the role that this modification plays in increasing antigenic diversity and circumventing tolerance mechanisms. Early published work demonstrated that citrullinated proteins are specifically targeted by autoantibodies in rheumatoid arthritis and that citrullinated peptides are more readily presented to T cells by arthritis-susceptible HLA class II 'shared epitope' proteins. Emerging data support the relevance of citrullinated epitopes in other autoimmune diseases, including type 1 diabetes and multiple sclerosis, whose susceptible HLA haplotypes also preferentially present citrullinated peptides. In these settings, autoimmune patients have been shown to have elevated responses to citrullinated epitopes derived from tissue-specific antigens. Contrasting evidence implicates autophagy or perforin and complement-mediated membrane attack as inducers of ectopic citrullination. In either case, the peptidyl deiminases responsible for citrullination are activated in response to inflammation or insult, providing a mechanistic link between this post-translational modification and interactions with the environment and infection. As such, it is likely that immune recognition of citrullinated epitopes also plays a role in pathogen clearance. Indeed, our recent data suggest that responses to citrullinated peptides facilitate recognition of novel influenza strains. Therefore, increased understanding of responses to citrullinated epitopes may provide important insights about the initiation of autoimmunity and recognition of heterologous viruses.
Keywords: T cells; antigens/peptides/epitopes; arthritis (including rheumatoid arthritis); autoimmunity; diabetes.
© 2016 John Wiley & Sons Ltd.
Figures

References
-
- Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol 2006; 38:1662–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

