ATP-dependent chromatin remodeling during mammalian development
- PMID: 27531948
- PMCID: PMC5004879
- DOI: 10.1242/dev.128892
ATP-dependent chromatin remodeling during mammalian development
Abstract
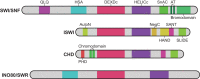
Precise gene expression ensures proper stem and progenitor cell differentiation, lineage commitment and organogenesis during mammalian development. ATP-dependent chromatin-remodeling complexes utilize the energy from ATP hydrolysis to reorganize chromatin and, hence, regulate gene expression. These complexes contain diverse subunits that together provide a multitude of functions, from early embryogenesis through cell differentiation and development into various adult tissues. Here, we review the functions of chromatin remodelers and their different subunits during mammalian development. We discuss the mechanisms by which chromatin remodelers function and highlight their specificities during mammalian cell differentiation and organogenesis.
Keywords: Chromatin remodeler; Chromatin remodeling; Differentiation; Gene expression; Transcription.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Alajem A., Biran A., Harikumar A., Sailaja B. S., Aaronson Y., Livyatan I., Nissim-Rafinia M., Sommer A. G., Mostoslavsky G., Gerbasi V. R. et al. (2015). Differential association of chromatin proteins identifies BAF60a/SMARCD1 as a regulator of embryonic stem cell differentiation. Cell Rep. 10, 2019-2031. 10.1016/j.celrep.2015.02.064 - DOI - PubMed
-
- Albini S., Coutinho Toto P., Dall'Agnese A., Malecova B., Cenciarelli C., Felsani A., Caruso M., Bultman S. J. and Puri P. L. (2015). Brahma is required for cell cycle arrest and late muscle gene expression during skeletal myogenesis. EMBO Rep. 16, 1037-1050. 10.15252/embr.201540159 - DOI - PMC - PubMed
-
- Alvarez-Saavedra M., De Repentigny Y., Lagali P. S., Raghu Ram E. V. S., Yan K., Hashem E., Ivanochko D., Huh M. S., Yang D., Mears A. J. et al. (2014). Snf2h-mediated chromatin organization and histone H1 dynamics govern cerebellar morphogenesis and neural maturation. Nat. Commun. 5, 4181 10.1038/ncomms5181 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

