Traumatic Brain Injury Activation of the Adult Subventricular Zone Neurogenic Niche
- PMID: 27531972
- PMCID: PMC4969304
- DOI: 10.3389/fnins.2016.00332
Traumatic Brain Injury Activation of the Adult Subventricular Zone Neurogenic Niche
Abstract
Traumatic brain injury (TBI) is common in both civilian and military life, placing a large burden on survivors and society. However, with the recognition of neural stem cells in adult mammals, including humans, came the possibility to harness these cells for repair of damaged brain, whereas previously this was thought to be impossible. In this review, we focus on the rodent adult subventricular zone (SVZ), an important neurogenic niche within the mature brain in which neural stem cells continue to reside. We review how the SVZ is perturbed following various animal TBI models with regards to cell proliferation, emigration, survival, and differentiation, and we review specific molecules involved in these processes. Together, this information suggests next steps in attempting to translate knowledge from TBI animal models into human therapies for TBI.
Keywords: adult neurogenesis; proliferation; regeneration; stem cells; traumatic brain injury (TBI).
Figures
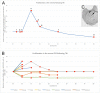
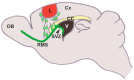
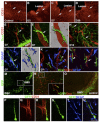
References
-
- Acosta S. A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D. M., et al. . (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS ONE 8:e53376. 10.1371/journal.pone.0053376 - DOI - PMC - PubMed
-
- Acosta S. A., Tajiri N., Shinozuka K., Ishikawa H., Sanberg P. R., Sanchez-Ramos J., et al. . (2014). Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS ONE 9:e90953. 10.1371/journal.pone.0090953 - DOI - PMC - PubMed
-
- Agrawal A., Timothy J., Pandit L., Manju M. (2006). Post-traumatic epilepsy: an overview. Clin. Neurol. Neurosurg. 108, 433–439. - PubMed
-
- Alonso G., Prieto M., Chauvet N. (1999). Tangential migration of young neurons arising from the subventricular zone of adult rats is impaired by surgical lesions passing through their natural migratory pathway. J. Comp. Neurol. 405, 508–528. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

