Recurrent Sleep Fragmentation Induces Insulin and Neuroprotective Mechanisms in Middle-Aged Flies
- PMID: 27531979
- PMCID: PMC4969361
- DOI: 10.3389/fnagi.2016.00180
Recurrent Sleep Fragmentation Induces Insulin and Neuroprotective Mechanisms in Middle-Aged Flies
Abstract
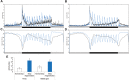
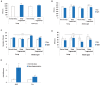
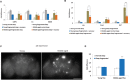
Lack of quality sleep increases central nervous system oxidative stress and impairs removal of neurotoxic soluble metabolites from brain parenchyma. During aging poor sleep quality, caused by sleep fragmentation, increases central nervous system cellular stress. Currently, it is not known how organisms offset age-related cytotoxic metabolite increases in order to safeguard neuronal survival. Furthermore, it is not understood how age and sleep fragmentation interact to affect oxidative stress protection pathways. We demonstrate sleep fragmentation increases systems that protect against oxidative damage and neuroprotective endoplasmic reticulum molecular chaperones, as well as neuronal insulin and dopaminergic expression in middle-aged Drosophila males. Interestingly, even after sleep recovery the expression of these genes was still upregulated in middle-aged flies. Finally, sleep fragmentation generates higher levels of reactive oxygen species (ROS) in middle-aged flies and after sleep recovery these levels remain significantly higher than in young flies. The fact that neuroprotective pathways remain upregulated in middle-aged flies beyond sleep fragmentation suggests it might represent a strong stressor for the brain during later life.
Keywords: Nrf2; dopamine; glucagon; insulin; metabolism; molecular chaperone; sleep.
Figures
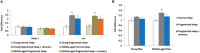
Similar articles
-
Blue light induces a neuroprotective gene expression program in Drosophila photoreceptors.BMC Neurosci. 2018 Jul 20;19(1):43. doi: 10.1186/s12868-018-0443-y. BMC Neurosci. 2018. PMID: 30029619 Free PMC article.
-
Hesperidin attenuates iron-induced oxidative damage and dopamine depletion in Drosophila melanogaster model of Parkinson's disease.Chem Biol Interact. 2018 Jan 5;279:177-186. doi: 10.1016/j.cbi.2017.11.018. Epub 2017 Dec 2. Chem Biol Interact. 2018. PMID: 29191452
-
Neuroprotective effects of PACAP against paraquat-induced oxidative stress in the Drosophila central nervous system.Hum Mol Genet. 2019 Jun 1;28(11):1905-1918. doi: 10.1093/hmg/ddz031. Hum Mol Genet. 2019. PMID: 30715303
-
Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling.Int J Mol Sci. 2017 Dec 22;19(1):26. doi: 10.3390/ijms19010026. Int J Mol Sci. 2017. PMID: 29271910 Free PMC article. Review.
-
Oxidative stress and protein aggregation during biological aging.Exp Gerontol. 2001 Sep;36(9):1539-50. doi: 10.1016/s0531-5565(01)00139-5. Exp Gerontol. 2001. PMID: 11525876 Review.
Cited by
-
Tired and stressed: Examining the need for sleep.Eur J Neurosci. 2020 Jan;51(1):494-508. doi: 10.1111/ejn.14197. Epub 2018 Oct 26. Eur J Neurosci. 2020. PMID: 30295966 Free PMC article. Review.
-
Nighttime caffeine intake increases motor impulsivity.iScience. 2025 Jul 24;28(8):113197. doi: 10.1016/j.isci.2025.113197. eCollection 2025 Aug 15. iScience. 2025. PMID: 40809001 Free PMC article.
-
Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila melanogaster Adults.Brain Sci. 2020 Jul 28;10(8):487. doi: 10.3390/brainsci10080487. Brain Sci. 2020. PMID: 32731370 Free PMC article.
-
Aging and the clock: Perspective from flies to humans.Eur J Neurosci. 2020 Jan;51(1):454-481. doi: 10.1111/ejn.14176. Epub 2018 Oct 30. Eur J Neurosci. 2020. PMID: 30269400 Free PMC article. Review.
-
Sleep mediates the association between homocysteine and oxidative status in mild cognitive impairment.Sci Rep. 2017 Aug 10;7(1):7719. doi: 10.1038/s41598-017-08292-4. Sci Rep. 2017. PMID: 28798397 Free PMC article.
References
-
- Broughton S. J., Piper M. D., Ikeya T., Bass T. M., Jacobson J., Driege Y., et al. (2005). Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. U.S.A. 102 3105–3110. 10.1073/pnas.0405775102 - DOI - PMC - PubMed
-
- Butovsky O., Koronyo-Hamaoui M., Kunis G., Ophir E., Landa G., Cohen H., et al. (2006). Glatiramer acetate fights against Alzheimer’s disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc. Natl. Acad. Sci. U.S.A. 103 11784–11789. 10.1073/pnas.0604681103 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

