"Oxygen Sensing" by Na,K-ATPase: These Miraculous Thiols
- PMID: 27531981
- PMCID: PMC4970491
- DOI: 10.3389/fphys.2016.00314
"Oxygen Sensing" by Na,K-ATPase: These Miraculous Thiols
Abstract
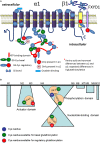
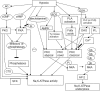
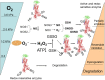
Control over the Na,K-ATPase function plays a central role in adaptation of the organisms to hypoxic and anoxic conditions. As the enzyme itself does not possess O2 binding sites its "oxygen-sensitivity" is mediated by a variety of redox-sensitive modifications including S-glutathionylation, S-nitrosylation, and redox-sensitive phosphorylation. This is an overview of the current knowledge on the plethora of molecular mechanisms tuning the activity of the ATP-consuming Na,K-ATPase to the cellular metabolic activity. Recent findings suggest that oxygen-derived free radicals and H2O2, NO, and oxidized glutathione are the signaling messengers that make the Na,K-ATPase "oxygen-sensitive." This very ancient signaling pathway targeting thiols of all three subunits of the Na,K-ATPase as well as redox-sensitive kinases sustains the enzyme activity at the "optimal" level avoiding terminal ATP depletion and maintaining the transmembrane ion gradients in cells of anoxia-tolerant species. We acknowledge the complexity of the underlying processes as we characterize the sources of reactive oxygen and nitrogen species production in hypoxic cells, and identify their targets, the reactive thiol groups which, upon modification, impact the enzyme activity. Structured accordingly, this review presents a summary on (i) the sources of free radical production in hypoxic cells, (ii) localization of regulatory thiols within the Na,K-ATPase and the role reversible thiol modifications play in responses of the enzyme to a variety of stimuli (hypoxia, receptors' activation) (iii) redox-sensitive regulatory phosphorylation, and (iv) the role of fine modulation of the Na,K-ATPase function in survival success under hypoxic conditions. The co-authors attempted to cover all the contradictions and standing hypotheses in the field and propose the possible future developments in this dynamic area of research, the importance of which is hard to overestimate. Better understanding of the processes underlying successful adaptation strategies will make it possible to harness them and use for treatment of patients with stroke and myocardial infarction, sleep apnoea and high altitude pulmonary oedema, and those undergoing surgical interventions associated with the interruption of blood perfusion.
Keywords: S-glutathionylation; S-nitrosylation; Sodium-Potassium-Exchanging ATPase; hypoxia; redox regulation; thiols.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

