Neuroimmune Interaction in the Regulation of Peripheral Opioid-Mediated Analgesia in Inflammation
- PMID: 27532001
- PMCID: PMC4970451
- DOI: 10.3389/fimmu.2016.00293
Neuroimmune Interaction in the Regulation of Peripheral Opioid-Mediated Analgesia in Inflammation
Abstract
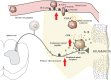
Peripheral immune cell-mediated analgesia in inflammation is an important endogenous mechanism of pain control. Opioid receptors localized on peripheral sensory nerve terminals are activated by endogenous opioid peptides released from immune cells to produce significant analgesia. Following transendothelial migration of opioid-containing leukocytes into peripheral sites of inflammation, opioid peptides are released into a harsh milieu associated with an increase in temperature, low pH, and high proteolytic activity. Together, this microenvironment has been suggested to increase the activity of opioid peptide metabolism. Therefore, the proximity of immune cells and nerve fibers may be essential to produce adequate analgesic effects. Close associations between opioid-containing immune cells and peripheral nerve terminals have been observed. However, it is not yet determined whether these immune cells actually form synaptic-like contacts with peripheral sensory terminals and/or whether they secrete opioids in a paracrine manner. This review will provide novel insight into the peripheral mechanisms of immune-derived analgesia in inflammation, in particular, the importance of direct interactions between immune cells and the peripheral nervous system.
Keywords: immune cells; inflammation; neuroimmune; opioids; pain; peripheral nervous system.
Figures

Similar articles
-
Involvement of the peripheral sensory and sympathetic nervous system in the vascular endothelial expression of ICAM-1 and the recruitment of opioid-containing immune cells to inhibit inflammatory pain.Brain Behav Immun. 2010 Nov;24(8):1310-23. doi: 10.1016/j.bbi.2010.06.008. Epub 2010 Jul 1. Brain Behav Immun. 2010. PMID: 20600813
-
Mechanisms of peripheral immune-cell-mediated analgesia in inflammation: clinical and therapeutic implications.Trends Pharmacol Sci. 2010 Sep;31(9):427-33. doi: 10.1016/j.tips.2010.05.008. Epub 2010 Jun 28. Trends Pharmacol Sci. 2010. PMID: 20591509 Review.
-
Targeting of opioid-producing leukocytes for pain control.Neuropeptides. 2007 Dec;41(6):355-63. doi: 10.1016/j.npep.2007.06.001. Epub 2007 Jul 20. Neuropeptides. 2007. PMID: 17640727 Review.
-
Peripherally acting opioids and clinical implications for pain control.Pain Physician. 2011 May-Jun;14(3):249-58. Pain Physician. 2011. PMID: 21587328 Review.
-
Opioids and sensory nerves.Handb Exp Pharmacol. 2009;(194):495-518. doi: 10.1007/978-3-540-79090-7_14. Handb Exp Pharmacol. 2009. PMID: 19655116 Review.
Cited by
-
Lymphocyte opioid receptors as innovative biomarkers of osteoarthritic pain, for the assessment and risk management of opioid tailored therapy, before hip surgery, to prevent chronic pain and opioid tolerance/addiction development: OpMarkArt (Opioids-Markers-Arthroprosthesis) study protocol for a randomized controlled trial.Trials. 2017 Dec 19;18(1):605. doi: 10.1186/s13063-017-2363-z. Trials. 2017. PMID: 29258584 Free PMC article. Clinical Trial.
-
Exploring the Versatility of Azine Derivatives: A Comprehensive Review on Synthesis and Biological Applications.Mini Rev Med Chem. 2025;25(6):425-439. doi: 10.2174/0113895575363243241129100845. Mini Rev Med Chem. 2025. PMID: 39810540 Free PMC article. Review.
-
Sex differences in effects of gestational polychlorinated biphenyl exposure on hypothalamic neuroimmune and neuromodulator systems in neonatal rats.Toxicol Appl Pharmacol. 2018 Aug 15;353:55-66. doi: 10.1016/j.taap.2018.06.002. Epub 2018 Jun 5. Toxicol Appl Pharmacol. 2018. PMID: 29879404 Free PMC article.
-
Peripherally Acting Opioids in Orofacial Pain.Front Neurosci. 2021 May 4;15:665445. doi: 10.3389/fnins.2021.665445. eCollection 2021. Front Neurosci. 2021. PMID: 34017236 Free PMC article. Review.
-
Neuroimmunology of cancer and associated symptomology.Immunol Cell Biol. 2021 Oct;99(9):949-961. doi: 10.1111/imcb.12496. Epub 2021 Sep 12. Immunol Cell Biol. 2021. PMID: 34355434 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

