Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial
- PMID: 27532364
- PMCID: PMC5063698
- DOI: 10.1001/jamaoncol.2016.1279
Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial
Erratum in
-
Missing Conflict of Interest Disclosure.JAMA Oncol. 2019 Jan 1;5(1):122. doi: 10.1001/jamaoncol.2018.5678. JAMA Oncol. 2019. PMID: 30422161 Free PMC article. No abstract available.
Abstract
Importance: Estrogen receptor α (ESR1) mutations found in metastatic breast cancer (MBC) promote ligand-independent receptor activation and resistance to estrogen-deprivation therapy in laboratory models. The prevalence of these mutations and their potential impact on clinical outcomes has not been established.
Objective: To determine the prevalence of ESR1 mutations (Y537S and D538G) in estrogen receptor (ER)-positive MBC and determine whether mutation is associated with inferior outcomes.
Design, setting, and participants: From December 16, 2014, to August 26, 2015, we analyzed cell-free DNA (cfDNA) from baseline plasma samples from participants in the BOLERO-2 double-blind phase 3 study that randomized patients from 189 centers in 24 countries with MBC to exemestane plus placebo or exemestane plus everolimus. The study enrolled postmenopausal women with a diagnosis of MBC and prior exposure to an aromatase inhibitor. Baseline plasma samples were available from 541 of 724 patients (74.7%). We assessed the effect of mutation on overall survival of the population and the effect of mutation on progression-free survival (PFS) by treatment arm.
Interventions: Patients were randomized to treatment with exemestane (25 mg oral daily) together with everolimus (10 mg oral daily) or with placebo.
Main outcomes and measures: The 2 most frequent mutations in ESR1 (Y537S and D538G) were analyzed from cfDNA using droplet digital polymerase chain reaction and samples scored as wild-type, D538G, Y537S, or double mutant. Cox-proportional hazards model was used to assess PFS in patient subgroups defined by mutations, and the effect of each mutation on overall survival.
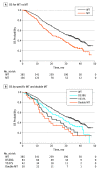
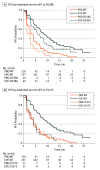
Results: Of 541 evaluable patients, 156 (28.8%) had ESR1 mutation D538G (21.1%) and/or Y537S (13.3%), and 30 had both. These mutations were associated with shorter overall survival (wild-type, 32.1 months [95% CI, 28.09-36.40 months]; D538G, 25.99 months [95% CI, 19.19-32.36 months]; Y537S, 19.98 months [13.01-29.31 months]; both mutations, 15.15 months [95% CI, 10.87-27.43 months]). The D538G group (hazard ratio, 0.34 [95% CI, 0.02-0.57]) derived a similar PFS benefit as wild type from addition of everolimus to exemestane.
Conclusions and relevance: ESR1 mutations are prevalent in ER-positive aromatase inhibitor-treated MBC. Both Y537S and D538G mutations are associated with more aggressive disease biology.
Trial registration: clinicaltrials.gov Identifier: NCT00863655.
Conflict of interest statement
Disclosures: Drs Chen, He, Patel, and Voi are employees of Novartis. Dr Chandarlapaty has received consulting fees from AstraZeneca. No other disclosures are reported.
Figures
Comment in
-
ESR1 Mutations in Cell-Free DNA of Breast Cancer: Predictive "Tip of the Iceberg".JAMA Oncol. 2016 Oct 1;2(10):1315-1316. doi: 10.1001/jamaoncol.2016.1268. JAMA Oncol. 2016. PMID: 27532824 Free PMC article. No abstract available.
-
Failure to Accurately Disclose Conflicts of Interest in Articles Published in JAMA Oncology.JAMA Oncol. 2019 Jan 1;5(1):118-119. doi: 10.1001/jamaoncol.2018.5674. JAMA Oncol. 2019. PMID: 30422162 No abstract available.
References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717. - PubMed
-
- Mouridsen H, Gershanovich M, Sun Y, et al. . Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21(11):2101-2109. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

