Modeling Mosquito-Borne Disease Spread in U.S. Urbanized Areas: The Case of Dengue in Miami
- PMID: 27532496
- PMCID: PMC4988691
- DOI: 10.1371/journal.pone.0161365
Modeling Mosquito-Borne Disease Spread in U.S. Urbanized Areas: The Case of Dengue in Miami
Abstract
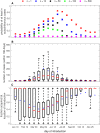
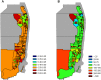
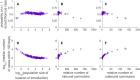
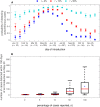
Expansion of mosquito-borne pathogens into more temperate regions of the world necessitates tools such as mathematical models for understanding the factors that contribute to the introduction and emergence of a disease in populations naïve to the disease. Often, these models are not developed and analyzed until after a pathogen is detected in a population. In this study, we develop a spatially explicit stochastic model parameterized with publicly available U.S. Census data for studying the potential for disease spread in Urbanized Areas of the United States. To illustrate the utility of the model, we specifically study the potential for introductions of dengue to lead to autochthonous transmission and outbreaks in a population representative of the Miami Urbanized Area, where introductions of dengue have occurred frequently in recent years. We describe seasonal fluctuations in mosquito populations by fitting a population model to trap data provided by the Miami-Dade Mosquito Control Division. We show that the timing and location of introduced cases could play an important role in determining both the probability that local transmission occurs as well as the total number of cases throughout the entire region following introduction. We show that at low rates of clinical presentation, small outbreaks of dengue could go completely undetected during a season, which may confound mitigation efforts that rely upon detection. We discuss the sensitivity of the model to several critical parameter values that are currently poorly characterized and motivate the collection of additional data to strengthen the predictive power of this and similar models. Finally, we emphasize the utility of the general structure of this model in studying mosquito-borne diseases such as chikungunya and Zika virus in other regions.
Conflict of interest statement
Figures
References
-
- Gubler DJ. Vector-borne diseases. Revue Scientifique et Technique (International Office of Epizootics). 2009. August;28(2):583–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

