Prolonged Exposure of Primary Human Muscle Cells to Plasma Fatty Acids Associated with Obese Phenotype Induces Persistent Suppression of Muscle Mitochondrial ATP Synthase β Subunit
- PMID: 27532680
- PMCID: PMC4988792
- DOI: 10.1371/journal.pone.0160057
Prolonged Exposure of Primary Human Muscle Cells to Plasma Fatty Acids Associated with Obese Phenotype Induces Persistent Suppression of Muscle Mitochondrial ATP Synthase β Subunit
Abstract
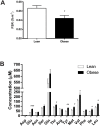
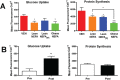
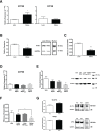
Our previous studies show reduced abundance of the β-subunit of mitochondrial H+-ATP synthase (β-F1-ATPase) in skeletal muscle of obese individuals. The β-F1-ATPase forms the catalytic core of the ATP synthase, and it is critical for ATP production in muscle. The mechanism(s) impairing β-F1-ATPase metabolism in obesity, however, are not completely understood. First, we studied total muscle protein synthesis and the translation efficiency of β-F1-ATPase in obese (BMI, 36±1 kg/m2) and lean (BMI, 22±1 kg/m2) subjects. Both total protein synthesis (0.044±0.006 vs 0.066±0.006%·h-1) and translation efficiency of β-F1-ATPase (0.0031±0.0007 vs 0.0073±0.0004) were lower in muscle from the obese subjects when compared to the lean controls (P<0.05). We then evaluated these same responses in a primary cell culture model, and tested the specific hypothesis that circulating non-esterified fatty acids (NEFA) in obesity play a role in the responses observed in humans. The findings on total protein synthesis and translation efficiency of β-F1-ATPase in primary myotubes cultured from a lean subject, and after exposure to NEFA extracted from serum of an obese subject, were similar to those obtained in humans. Among candidate microRNAs (i.e., non-coding RNAs regulating gene expression), we identified miR-127-5p in preventing the production of β-F1-ATPase. Muscle expression of miR-127-5p negatively correlated with β-F1-ATPase protein translation efficiency in humans (r = - 0.6744; P<0.01), and could be modeled in vitro by prolonged exposure of primary myotubes derived from the lean subject to NEFA extracted from the obese subject. On the other hand, locked nucleic acid inhibitor synthesized to target miR-127-5p significantly increased β-F1-ATPase translation efficiency in myotubes (0.6±0.1 vs 1.3±0.3, in control vs exposure to 50 nM inhibitor; P<0.05). Our experiments implicate circulating NEFA in obesity in suppressing muscle protein metabolism, and establish impaired β-F1-ATPase translation as an important consequence of obesity.
Conflict of interest statement
Figures



References
-
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289: 76–79. - PubMed
-
- Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10: 142–148. - PubMed
-
- Katsanos CS, Mandarino LJ. Protein metabolism in human obesity: a shift in focus from whole-body to skeletal muscle. Obesity (Silver Spring). 2011;19: 469–475. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

