Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia
- PMID: 27532807
- PMCID: PMC4994343
- DOI: 10.3201/eid2209.151164
Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia
Abstract
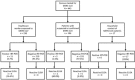
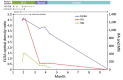
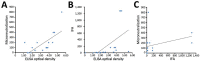
We explored the feasibility of collecting convalescent plasma for passive immunotherapy of Middle East respiratory syndrome coronavirus (MERS-CoV) infection by using ELISA to screen serum samples from 443 potential plasma donors: 196 patients with suspected or laboratory-confirmed MERS-CoV infection, 230 healthcare workers, and 17 household contacts exposed to MERS-CoV. ELISA-reactive samples were further tested by indirect fluorescent antibody and microneutralization assays. Of the 443 tested samples, 12 (2.7%) had a reactive ELISA result, and 9 of the 12 had reactive indirect fluorescent antibody and microneutralization assay titers. Undertaking clinical trials of convalescent plasma for passive immunotherapy of MERS-CoV infection may be feasible, but such trials would be challenging because of the small pool of potential donors with sufficiently high antibody titers. Alternative strategies to identify convalescent plasma donors with adequate antibody titers should be explored, including the sampling of serum from patients with more severe disease and sampling at earlier points during illness.
Keywords: ELISA; IFA; MERS; MERS-CoV; Middle East respiratory syndrome; Middle East respiratory syndrome coronavirus; Saudi Arabia; antibody titers; convalescent phase; convalescent plasma; convalescent-phase plasma; feasibility study; humans; immunotherapy; indirect immunofluorescent antibody assay; intensive care; microneutralization; neutralizing antibodies; respiratory infections; seroreactive; viruses.
Figures
References
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) [cited 2016 Jun 26]. http://www.who.int/emergencies/mers-cov/en/
-
- Public Health England, International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). Treatment of MERS-CoV: information for clinicians. Clinical decision-making support for treatment of MERS-CoV patients. V3.0 [cited 2016 Apr 29]. http://www.gov.uk/government/uploads/system/uploads/attachment_data/file...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

