Efficient Generation of Viral and Integration-Free Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes
- PMID: 27532816
- PMCID: PMC5087818
- DOI: 10.1002/cpsc.11
Efficient Generation of Viral and Integration-Free Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes
Abstract
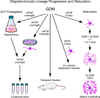
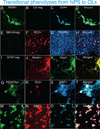
Here we document three highly reproducible protocols: (1) a culture system for the derivation of human oligodendrocytes (OLs) from human induced pluripotent stem cells (hiPS) and their further maturation-our protocol generates viral- and integration-free OLs that efficiently commit and move forward in the OL lineage, recapitulating all the steps known to occur during in vivo development; (2) a method for the isolation, propagation and maintenance of neural stem cells (NSCs); and (3) a protocol for the production, isolation, and maintenance of OLs from perinatal rodent and human brain-derived NSCs. Our unique culture systems rely on a series of chemically defined media, specifically designed and carefully characterized for each developmental stage of OL as they advance from OL progenitors to mature, myelinating cells. We are confident that these protocols bring our field a step closer to efficient autologous cell replacement therapies and disease modeling. © 2016 by John Wiley & Sons, Inc.
Keywords: NSC; chemically defined media; human induced pluripotent stem cells; lineage progression; neural stem cells; neurospheres; oligodendrocyte maturation; oligodendrocyte specification; oligospheres.
Copyright © 2016 John Wiley & Sons, Inc.
Conflict of interest statement
The authors acknowledge no conflict of interest
Figures





Similar articles
-
Efficient Generation of Viral and Integration-Free Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes.Curr Protoc Stem Cell Biol. 2016 Nov;39(1):2D.18.1-2D.18.28. doi: 10.1002/cpsc.19. Curr Protoc Stem Cell Biol. 2016. PMID: 31816188
-
Culture system for rodent and human oligodendrocyte specification, lineage progression, and maturation.Curr Protoc Stem Cell Biol. 2009 Sep;Chapter 2:Unit 2D.4. doi: 10.1002/9780470151808.sc02d04s10. Curr Protoc Stem Cell Biol. 2009. PMID: 19725014
-
Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells.Stem Cells Transl Med. 2013 Nov;2(11):862-70. doi: 10.5966/sctm.2013-0080. Epub 2013 Oct 10. Stem Cells Transl Med. 2013. PMID: 24113065 Free PMC article.
-
Identity and Maturity of iPSC-Derived Oligodendrocytes in 2D and Organoid Systems.Cells. 2024 Apr 13;13(8):674. doi: 10.3390/cells13080674. Cells. 2024. PMID: 38667289 Free PMC article. Review.
-
Establishment and properties of neural stem cell clones: plasticity in vitro and in vivo.Brain Pathol. 1999 Jul;9(3):569-98. doi: 10.1111/j.1750-3639.1999.tb00542.x. Brain Pathol. 1999. PMID: 10416994 Free PMC article. Review.
Cited by
-
Oligodendrocyte Progenitors Display Enhanced Proliferation and Autophagy after Space Flight.Biomolecules. 2023 Jan 19;13(2):201. doi: 10.3390/biom13020201. Biomolecules. 2023. PMID: 36830573 Free PMC article.
-
Trophic factors are essential for the survival of grafted oligodendrocyte progenitors and for neuroprotection after perinatal excitotoxicity.Neural Regen Res. 2020 Mar;15(3):557-568. doi: 10.4103/1673-5374.266066. Neural Regen Res. 2020. PMID: 31571668 Free PMC article.
-
Metabolomics Profile of the Secretome of Space-Flown Oligodendrocytes.Cells. 2023 Sep 11;12(18):2249. doi: 10.3390/cells12182249. Cells. 2023. PMID: 37759473 Free PMC article.
-
Physiologically-Relevant Cellular Models for Biomarkers and Drug Discovery.Ann Stem Cell Res Ther. 2018;2(2):1014. Epub 2018 Apr 26. Ann Stem Cell Res Ther. 2018. PMID: 34485994 Free PMC article. No abstract available.
-
Human Neural Stem Cells Flown into Space Proliferate and Generate Young Neurons.Appl Sci (Basel). 2019 Oct 1;9(19):4042. doi: 10.3390/app9194042. Epub 2019 Sep 27. Appl Sci (Basel). 2019. PMID: 34484810 Free PMC article.
References
-
- Biancotti JC, Lavon N. Derivation, expansion, and characterization of human embryonic stem cell lines from aneuploid embryos. Methods in Molecular Biology (Clifton, N.J.) 2012;873:163–178. - PubMed
-
- Bjugstad KB, Redmond DE, Jr, Teng YD, Elsworth JD, Roth RH, Blanchard BC, Snyder EY, Sladek JR., Jr Neural stem cells implanted into MPTP-treated monkeys increase the size of endogenous tyrosine hydroxylase-positive cells found in the striatum: a return to control measures. Cell Transplant. 2005;14(4):183–192. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

