Therapy-Emergent Drug Resistance to Integrase Strand Transfer Inhibitors in HIV-1 Patients: A Subgroup Meta-Analysis of Clinical Trials
- PMID: 27532886
- PMCID: PMC4988762
- DOI: 10.1371/journal.pone.0160087
Therapy-Emergent Drug Resistance to Integrase Strand Transfer Inhibitors in HIV-1 Patients: A Subgroup Meta-Analysis of Clinical Trials
Abstract
Background: Integrase strand transfer inhibitors (INSTIs) are a novel class of anti-HIV agents that show high activity in inhibiting HIV-1 replication. Currently, licensed INSTIs include raltegravir (RAL), elvitegravir (EVG) and dolutegravir (DTG); these drugs have played a critical role in AIDS therapy, serving as additional weapons in the arsenal for treating patients infected with HIV-1. To date, long-term data regarding clinical experience with INSTI use and the emergence of resistance remain scarce. However, the literature is likely now sufficiently comprehensive to warrant a meta-analysis of resistance to INSTIs.
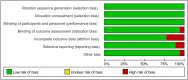
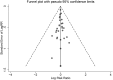
Methods: Our team implemented a manuscript retrieval protocol using Medical Subject Headings (MeSH) via the Web of Science, MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials databases. We screened the literature based on inclusion and exclusion criteria and then performed a quality analysis and evaluation using RevMan software, Stata software, and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). We also performed a subgroup analysis. Finally, we calculated resistance rates and risk ratios (RRs) for the three types of drugs.
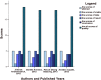
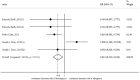
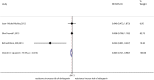
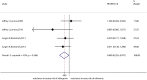
Results: We identified 26 references via the database search. A meta-analysis of the RAL data revealed that the resistance rate was 3.9% (95% CI = 2.9%-4.9%) for the selected randomized controlled trials (RCTs). However, the RAL resistance rate reached 40.9% (95% CI = 8.8%-72.9%) for the selected observational studies (OBSs). The rates of resistance to RAL that were associated with HIV subtypes A, B, and C as well as with more complex subtypes were 0.1% (95% CI = -0.7%-0.9%), 2.5% (95% CI = 0.5%-4.5%), 4.6% (95% CI = 2.7%-6.6%) and 2.2% (95% CI = 0.7%-3.7%), respectively. The rates of resistance to EVG and DTG were 1.2% (95% CI = 0.2%-2.2%) and 0.1% (95% CI = -0.2%-0.5%), respectively. Furthermore, we found that the RRs for antiviral resistance were 0.414 (95% CI = 0.210-0.816) between DTG and RAL and 0.499 (95% CI = 0.255-0.977) between EVG and RAL. When RAL was separately co-administered with nuclear nucleoside reverse transcriptase inhibitors (NRTIs) or protease inhibitors (PIs), the rates of resistance to RAL were 0.2% (95% CI = -0.1%-0.5%) and 0.2% (95% CI = -0.2%-0.6%), respectively. The ten major integrase mutations (including N155H, Y143C/R, Q148H/R, Y143Y/H, L74L/M, E92Q, E138E/A, Y143C, Q148Q and Y143S) can reduce the sensitivity of RAL and EVG. The resistance of DTG is mainly shown in 13 integrase mutations (including T97T/A, E138E/D, V151V/I, N155H, Q148, Y143C/H/R, T66A and E92Q).
Conclusions: Our results reveal that the DTG resistance rate was lower than the RAL resistance rate in a head-to-head comparison. Moreover, we confirmed that the EVG resistance rate was lower than the RAL resistance rate. In addition, our results revealed that the resistance rate of RAL was lower than that of efavirenz. The rates of resistance to RAL, EVG and DTG were specifically 3.9%, 1.2% and 0.1%, respectively. Compared with other types of antiviral drugs, the rates of resistance to INSTIs are generally lower. Unfortunately, the EVG and DTG resistance rates could not be compared because of a lack of data.
Conflict of interest statement
Figures






Similar articles
-
Effects of raltegravir or elvitegravir resistance signature mutations on the barrier to dolutegravir resistance in vitro.Antimicrob Agents Chemother. 2015 May;59(5):2596-606. doi: 10.1128/AAC.04844-14. Epub 2015 Feb 17. Antimicrob Agents Chemother. 2015. PMID: 25691633 Free PMC article.
-
The Combination of the R263K and T66I Resistance Substitutions in HIV-1 Integrase Is Incompatible with High-Level Viral Replication and the Development of High-Level Drug Resistance.J Virol. 2015 Nov;89(22):11269-74. doi: 10.1128/JVI.01881-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311878 Free PMC article.
-
Dolutegravir-Selected HIV-1 Containing the N155H and R263K Resistance Substitutions Does Not Acquire Additional Compensatory Mutations under Drug Pressure That Lead to Higher-Level Resistance and Increased Replicative Capacity.J Virol. 2015 Oct;89(20):10482-8. doi: 10.1128/JVI.01725-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246578 Free PMC article.
-
Meta-analysis and systematic review of the efficacy and resistance for human immunodeficiency virus type 1 integrase strand transfer inhibitors.Int J Antimicrob Agents. 2019 Nov;54(5):547-555. doi: 10.1016/j.ijantimicag.2019.08.008. Epub 2019 Aug 6. Int J Antimicrob Agents. 2019. PMID: 31398480
-
HIV-1 integrase strand transfer inhibitors: a review of current drugs, recent advances and drug resistance.Int J Antimicrob Agents. 2021 May;57(5):106343. doi: 10.1016/j.ijantimicag.2021.106343. Epub 2021 Apr 11. Int J Antimicrob Agents. 2021. PMID: 33852932 Review.
Cited by
-
Antiretrovirals for Prophylaxis Against COVID-19: A Comprehensive Literature Review.J Clin Pharmacol. 2021 May;61(5):581-590. doi: 10.1002/jcph.1788. Epub 2020 Dec 6. J Clin Pharmacol. 2021. PMID: 33217030 Free PMC article. Review.
-
An Isoquinoline Scaffold as a Novel Class of Allosteric HIV-1 Integrase Inhibitors.ACS Med Chem Lett. 2019 Jan 30;10(2):215-220. doi: 10.1021/acsmedchemlett.8b00633. eCollection 2019 Feb 14. ACS Med Chem Lett. 2019. PMID: 30783506 Free PMC article.
-
Adult antiretroviral therapy guidelines 2017.South Afr J HIV Med. 2017 Jul 15;18(1):776. doi: 10.4102/sajhivmed.v18i1.776. eCollection 2017. South Afr J HIV Med. 2017. PMID: 29568644 Free PMC article.
-
Dolutegravir based antiretroviral therapy compared to other combined antiretroviral regimens for the treatment of HIV-infected naive patients: A systematic review and meta-analysis.PLoS One. 2019 Sep 10;14(9):e0222229. doi: 10.1371/journal.pone.0222229. eCollection 2019. PLoS One. 2019. PMID: 31504060 Free PMC article.
-
Influence of the amino-terminal sequence on the structure and function of HIV integrase.Retrovirology. 2020 Aug 31;17(1):28. doi: 10.1186/s12977-020-00537-x. Retrovirology. 2020. PMID: 32867805 Free PMC article.
References
-
- Lopez-Cortes LF, Viciana P, Giron-Gonzalez JA, Romero-Palacios A, Marquez-Solero M, Martinez-Perez MA, et al. Clinical and virological efficacy of etravirine plus two active nucleos(t)ide analogs in an heterogeneous HIV-infected population. Plos One. 2014; 9: e97262 10.1371/journal.pone.0097262 - DOI - PMC - PubMed
-
- Manfredi R, Sabbatani S. A novel antiretroviral class (fusion inhibitors) in the management of HIV infection. Present features and future perspectives of enfuvirtide (T-20). Curr Med Chem. 2006; 13: 2369–2384. - PubMed
-
- Zolopa AR, Berger DS, Lampiris H, Zhong LJ, Chuck SL, Enejosa JV, et al. Activity of elvitegravir, a once-daily integrase inhibitor, against resistant HIV type 1: results of a phase 2, randomized, controlled, dose-ranging clinical trial. J Infect Dis. 2010; 201: 814–822. 10.1086/650698 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

