Targeting HER3 using mono- and bispecific antibodies or alternative scaffolds
- PMID: 27532938
- PMCID: PMC5058629
- DOI: 10.1080/19420862.2016.1212147
Targeting HER3 using mono- and bispecific antibodies or alternative scaffolds
Abstract
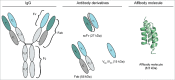
The human epidermal growth factor receptor 3 (HER3) has in recent years been recognized as a key node in the complex signaling network of many different cancers. It is implicated in de novo and acquired resistance against therapies targeting other growth factor receptors, e.g., EGFR, HER2, and it is a major activator of the PI3K/Akt signaling pathway. Consequently, HER3 has attracted substantial attention, and is today a key target for drugs in clinical development. Sophisticated protein engineering approaches have enabled the generation of a range of different affinity proteins targeting this receptor, including antibodies and alternative scaffolds that are either mono- or bispecific. Here, we describe HER3 and its role as a key tumor target, and give a comprehensive review of HER3-targeted proteins currently in development, including discussions on the opportunities and challenges of targeting this receptor.
Keywords: Affibody molecules; ErbB3; HER3; alternative scaffolds; bispecific antibodies; monoclonal antibodies; protein therapeutics; tumor targeting.
Figures

Similar articles
-
Engineering of a bispecific affibody molecule towards HER2 and HER3 by addition of an albumin-binding domain allows for affinity purification and in vivo half-life extension.Biotechnol J. 2014 Sep;9(9):1215-22. doi: 10.1002/biot.201400009. Epub 2014 Apr 23. Biotechnol J. 2014. PMID: 24678002
-
Antibody targeting of HER2/HER3 signaling overcomes heregulin-induced resistance to PI3K inhibition in prostate cancer.Int J Cancer. 2015 Jul 15;137(2):267-77. doi: 10.1002/ijc.29378. Epub 2014 Dec 19. Int J Cancer. 2015. PMID: 25471734
-
HER3/ErbB3, an emerging cancer therapeutic target.Acta Biochim Biophys Sin (Shanghai). 2016 Jan;48(1):39-48. doi: 10.1093/abbs/gmv103. Epub 2015 Oct 24. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 26496898 Review.
-
HER3, serious partner in crime: therapeutic approaches and potential biomarkers for effect of HER3-targeting.Pharmacol Ther. 2014 Jul;143(1):1-11. doi: 10.1016/j.pharmthera.2014.01.005. Epub 2014 Feb 7. Pharmacol Ther. 2014. PMID: 24513440 Review.
-
HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer.Cells. 2023 Oct 25;12(21):2517. doi: 10.3390/cells12212517. Cells. 2023. PMID: 37947595 Free PMC article. Review.
Cited by
-
Phage Display Derived Monoclonal Antibodies: From Bench to Bedside.Front Immunol. 2020 Aug 28;11:1986. doi: 10.3389/fimmu.2020.01986. eCollection 2020. Front Immunol. 2020. PMID: 32983137 Free PMC article. Review.
-
Novel functional anti-HER3 monoclonal antibodies with potent anti-cancer effects on various human epithelial cancers.Oncotarget. 2020 Jan 7;11(1):31-45. doi: 10.18632/oncotarget.27414. eCollection 2020 Jan 7. Oncotarget. 2020. PMID: 32002122 Free PMC article.
-
HER3-targeted therapy: the mechanism of drug resistance and the development of anticancer drugs.Cancer Drug Resist. 2024 Apr 29;7:14. doi: 10.20517/cdr.2024.11. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38835349 Free PMC article. Review.
-
Affibody molecules as engineered protein drugs.Exp Mol Med. 2017 Mar 24;49(3):e306. doi: 10.1038/emm.2017.35. Exp Mol Med. 2017. PMID: 28336959 Free PMC article. Review.
-
The emerging treatment landscape of targeted therapy in non-small-cell lung cancer.Signal Transduct Target Ther. 2019 Dec 17;4:61. doi: 10.1038/s41392-019-0099-9. eCollection 2019. Signal Transduct Target Ther. 2019. PMID: 31871778 Free PMC article. Review.
References
-
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:22437872; http://dx.doi.org/10.1038/nrc3236 - DOI - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127-37; PMID:11252954; http://dx.doi.org/10.1038/35052073 - DOI - PubMed
-
- Zahnow CA. ErbB receptors and their ligands in the breast. Expert Rev Mol Med 2006; 8:1-21; PMID:16984691; http://dx.doi.org/10.1017/S146239940600010X - DOI - PubMed
-
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 2009; 9:463-75; PMID:19536107; http://dx.doi.org/10.1038/nrc2656 - DOI - PubMed
-
- Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012; 12:553-63; PMID:22785351; http://dx.doi.org/10.1038/nrc3309 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
