The TRPM2 ion channel is required for sensitivity to warmth
- PMID: 27533035
- PMCID: PMC5720344
- DOI: 10.1038/nature19074
The TRPM2 ion channel is required for sensitivity to warmth
Abstract
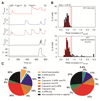
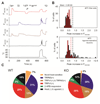
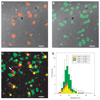
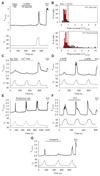
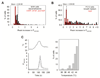
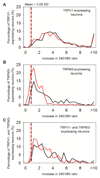
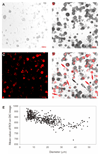
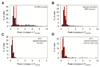
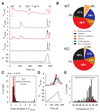
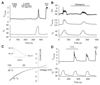
Thermally activated ion channels are known to detect the entire thermal range from extreme heat (TRPV2), painful heat (TRPV1, TRPM3 and ANO1), non-painful warmth (TRPV3 and TRPV4) and non-painful coolness (TRPM8) through to painful cold (TRPA1). Genetic deletion of each of these ion channels, however, has only modest effects on thermal behaviour in mice, with the exception of TRPM8, the deletion of which has marked effects on the perception of moderate coolness in the range 10-25 °C. The molecular mechanism responsible for detecting non-painful warmth, in particular, is unresolved. Here we used calcium imaging to identify a population of thermally sensitive somatosensory neurons which do not express any of the known thermally activated TRP channels. We then used a combination of calcium imaging, electrophysiology and RNA sequencing to show that the ion channel generating heat sensitivity in these neurons is TRPM2. Autonomic neurons, usually thought of as exclusively motor, also express TRPM2 and respond directly to heat. Mice in which TRPM2 had been genetically deleted showed a striking deficit in their sensation of non-noxious warm temperatures, consistent with the idea that TRPM2 initiates a 'warm' signal which drives cool-seeking behaviour.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

