Proteomic identification of cyclophilin A as a potential biomarker and therapeutic target in oral submucous fibrosis
- PMID: 27533088
- PMCID: PMC5312388
- DOI: 10.18632/oncotarget.11254
Proteomic identification of cyclophilin A as a potential biomarker and therapeutic target in oral submucous fibrosis
Abstract
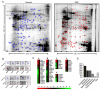
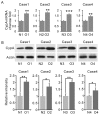
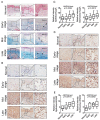
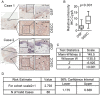
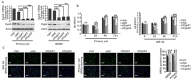
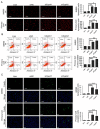
Oral submucous fibrosis (OSF) is a pre-cancerous lesion, which is characterized by fibrosis of the oral submucosa. Despite large body of studies focusing on this disease, the molecular mechanisms underlying the progression of OSF remained unclear. In this study, 2-DE-based proteomic approaches were employed to identify the differently expressed proteins between OSF and normal tissues. In total, 88 proteins were identified with altered expression levels, including CypA. Upregulation of CypA was further validated through immunohistochemistry staining combined with Q-PCR and western blot by using clinical samples. Statistical analyses reveal that CypA expression level is correlated to the progression of OSF. Finally, functional study reveals a pro-proliferative property of CypA in fibroblast cells by using multiple in vitro models. The present data suggest that CypA might be a potential biomarker and therapeutic target for OSF, and will lead to a better understanding of OSF pathogenesis.
Keywords: CypA; fibroblast; oral submucous fibrosis; proteomics.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures

Similar articles
-
Cyclophilin A was revealed as a candidate marker for human oral submucous fibrosis by proteomic analysis.Cancer Biomark. 2017 Sep 7;20(3):345-356. doi: 10.3233/CBM-170142. Cancer Biomark. 2017. PMID: 28826174
-
MALDI imaging reveals NCOA7 as a potential biomarker in oral squamous cell carcinoma arising from oral submucous fibrosis.Oncotarget. 2016 Sep 13;7(37):59987-60004. doi: 10.18632/oncotarget.11046. Oncotarget. 2016. PMID: 27509054 Free PMC article.
-
Quantitative proteomic analysis for novel biomarkers of buccal squamous cell carcinoma arising in background of oral submucous fibrosis.BMC Cancer. 2016 Aug 2;16:584. doi: 10.1186/s12885-016-2650-1. BMC Cancer. 2016. PMID: 27485544 Free PMC article.
-
Malignant transformation of oral submucous fibrosis: overview of histopathological aspects.Oral Surg Oral Med Oral Pathol Oral Radiol. 2016 Aug;122(2):200-9. doi: 10.1016/j.oooo.2015.11.024. Epub 2016 Apr 19. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016. PMID: 27422418 Review.
-
Molecular Pathology of Malignant Transformation of Oral Submucous Fibrosis.J Environ Pathol Toxicol Oncol. 2016;35(3):193-205. doi: 10.1615/JEnvironPatholToxicolOncol.2016014024. J Environ Pathol Toxicol Oncol. 2016. PMID: 27910776 Review.
Cited by
-
Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy.Int J Mol Sci. 2019 Jun 16;20(12):2940. doi: 10.3390/ijms20122940. Int J Mol Sci. 2019. PMID: 31208114 Free PMC article. Review.
-
[Expression of cyclophilin A in oral squamous cell carcinoma and its effect on cell proliferation and invasion].Hua Xi Kou Qiang Yi Xue Za Zhi. 2021 Apr 1;39(2):164-169. doi: 10.7518/hxkq.2021.02.006. Hua Xi Kou Qiang Yi Xue Za Zhi. 2021. PMID: 33834670 Free PMC article. Chinese.
-
Integrated Proteomics Based on 2D Gel Electrophoresis and Mass Spectrometry with Validations: Identification of a Biomarker Compendium for Oral Submucous Fibrosis-An Indian Study.J Pers Med. 2022 Feb 3;12(2):208. doi: 10.3390/jpm12020208. J Pers Med. 2022. PMID: 35207696 Free PMC article.
-
A contemporary narrative review to guide molecular epidemiology of oral submucous fibrosis.Int J Mol Epidemiol Genet. 2021 Aug 15;12(4):61-70. eCollection 2021. Int J Mol Epidemiol Genet. 2021. PMID: 34552689 Free PMC article. Review.
-
Molecular Genomics of Oral Submucous Fibrosis: A Narrative Review.Genes (Basel). 2025 May 22;16(6):612. doi: 10.3390/genes16060612. Genes (Basel). 2025. PMID: 40565504 Free PMC article. Review.
References
-
- Cox SC, Walker DM. Oral submucous fibrosis. A review. Australian dental journal. 1996;41:294–299. - PubMed
-
- Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: review on aetiology and pathogenesis. Oral oncology. 2006;42:561–568. - PubMed
-
- Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncology. 2006;42:561–568. - PubMed
-
- Murti P, Bhonsle R, Gupta P, Daftary D, Pindborg J, Mehta FS. Etiology of oral submucous fibrosis with special reference to the role of areca nut chewing. Journal of oral pathology & medicine. 1995;24:145–152. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

