Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice
- PMID: 27533619
- PMCID: PMC5074849
- DOI: 10.1002/hep.28774
Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice
Abstract
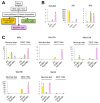
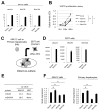
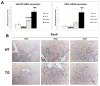
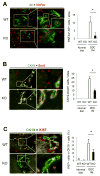
Hepatic repair is directed chiefly by the proliferation of resident mature epithelial cells. Furthermore, if predominant injury is to cholangiocytes, the hepatocytes can transdifferentiate to cholangiocytes to assist in the repair and vice versa, as shown by various fate-tracing studies. However, the molecular bases of reprogramming remain elusive. Using two models of biliary injury where repair occurs through cholangiocyte proliferation and hepatocyte transdifferentiation to cholangiocytes, we identify an important role of Wnt signaling. First we identify up-regulation of specific Wnt proteins in the cholangiocytes. Next, using conditional knockouts of Wntless and Wnt coreceptors low-density lipoprotein-related protein 5/6, transgenic mice expressing stable β-catenin, and in vitro studies, we show a role of Wnt signaling through β-catenin in hepatocyte to biliary transdifferentiation. Last, we show that specific Wnts regulate cholangiocyte proliferation, but in a β-catenin-independent manner.
Conclusion: Wnt signaling regulates hepatobiliary repair after cholestatic injury in both β-catenin-dependent and -independent manners. (Hepatology 2016;64:1652-1666).
© 2016 by the American Association for the Study of Liver Diseases.
Figures



Similar articles
-
β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation!Hepatology. 2014 Sep;60(3):964-76. doi: 10.1002/hep.27082. Epub 2014 Jul 25. Hepatology. 2014. PMID: 24700412 Free PMC article.
-
Long noncoding RNAs associated with liver regeneration 1 accelerates hepatocyte proliferation during liver regeneration by activating Wnt/β-catenin signaling.Hepatology. 2013 Aug;58(2):739-51. doi: 10.1002/hep.26361. Epub 2013 Jul 1. Hepatology. 2013. PMID: 23483581
-
WNT5A inhibits hepatocyte proliferation and concludes β-catenin signaling in liver regeneration.Am J Pathol. 2015 Aug;185(8):2194-205. doi: 10.1016/j.ajpath.2015.04.021. Epub 2015 Jun 19. Am J Pathol. 2015. PMID: 26100214 Free PMC article.
-
Transcription dynamics in a physiological process: β-catenin signaling directs liver metabolic zonation.Int J Biochem Cell Biol. 2011 Feb;43(2):271-8. doi: 10.1016/j.biocel.2009.11.004. Epub 2009 Nov 13. Int J Biochem Cell Biol. 2011. PMID: 19914393 Review.
-
The Wnt/β-catenin signaling pathway in liver biology and disease.Expert Rev Gastroenterol Hepatol. 2010 Dec;4(6):745-56. doi: 10.1586/egh.10.74. Expert Rev Gastroenterol Hepatol. 2010. PMID: 21108594 Free PMC article. Review.
Cited by
-
Wnt-β-catenin in hepatobiliary homeostasis, injury, and repair.Hepatology. 2023 Dec 1;78(6):1907-1921. doi: 10.1097/HEP.0000000000000495. Epub 2023 May 30. Hepatology. 2023. PMID: 37246413 Free PMC article. Review.
-
Microenvironmental control of the ductular reaction: balancing repair and disease progression.Cell Death Dis. 2025 Apr 4;16(1):246. doi: 10.1038/s41419-025-07590-4. Cell Death Dis. 2025. PMID: 40180915 Free PMC article. Review.
-
Tetrahedral framework nucleic acids ameliorate cholestatic liver disease by activating Wnt/β-catenin signaling and promoting ERK1/2 phosphorylation.Regen Biomater. 2025 Mar 20;12:rbaf017. doi: 10.1093/rb/rbaf017. eCollection 2025. Regen Biomater. 2025. PMID: 40385130 Free PMC article.
-
Fibroinflammatory Liver Injuries as Preneoplastic Condition in Cholangiopathies.Int J Mol Sci. 2018 Dec 4;19(12):3875. doi: 10.3390/ijms19123875. Int J Mol Sci. 2018. PMID: 30518128 Free PMC article. Review.
-
Increased Expression of Adherens Junction Components in Mouse Liver following Bile Duct Ligation.Biomolecules. 2019 Oct 22;9(10):636. doi: 10.3390/biom9100636. Biomolecules. 2019. PMID: 31652629 Free PMC article.
References
-
- Poupon R, Chazouilleres O, Poupon RE. Chronic cholestatic diseases. J Hepatol. 2000;32:129–140. - PubMed

