The efficacy of anti-PD-1 agents in acral and mucosal melanoma
- PMID: 27533633
- PMCID: PMC5134420
- DOI: 10.1002/cncr.30259
The efficacy of anti-PD-1 agents in acral and mucosal melanoma
Abstract
Background: Therapeutic antibodies against programmed cell death receptor 1 (PD-1) are considered front-line therapy in metastatic melanoma. The efficacy of PD-1 blockade for patients with biologically distinct melanomas arising from acral and mucosal surfaces has not been well described.
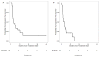
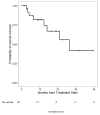
Methods: A multi-institutional, retrospective cohort analysis identified adults with advanced acral and mucosal melanoma who received treatment with nivolumab or pembrolizumab as standard clinical practice through expanded access programs or published prospective trials. Objective responses were determined using investigator-assessed Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Progression-free survival and overall survival were assessed using the Kaplan-Meier method.
Results: Sixty individuals were identified, including 25 (42%) with acral melanoma and 35 (58%) with mucosal melanoma. Fifty-one patients (85%) had received previous therapy, including 77% who had previously received ipilimumab. Forty patients (67%) received pembrolizumab at a dose of 2 mg/kg or 10 mg/kg, and 20 (33%) received nivolumab at a doses ranging from 0.3 to 10 mg/kg every 2 to 3 weeks. The objective response rate was 32% (95% confidence interval, 15%-54%) in patients with acral melanoma and 23% (95% confidence interval, 10%-40%) in those with mucosal melanoma. After a median follow-up of 20 months in the acral melanoma group and 10.6 months in the mucosal melanoma group, the median progression-free survival was 4.1 months and 3.9 months, respectively. Only 2 patients (3%) discontinued treatment because of toxicity.
Conclusions: Response rates to PD-1 blockade in patients with acral and mucosal melanomas were comparable to the published rates in patients with cutaneous melanoma and support the routine use of PD-1 blockade in clinical practice. Further investigation is needed to identify the mechanisms of response and resistance to therapy in these subtypes. Cancer 2016;122:3354-3362. © 2016 American Cancer Society.
Keywords: acral melanoma; anti-programmed cell death receptor 1 (anti-PD-1); immunotherapy; mucosal melanoma; nivolumab; pembrolizumab.
© 2016 American Cancer Society.
Conflict of interest statement
ANS received travel support from BMS and is on a scientific advisory board for Vaccinex. RRM received travel grants from Merck Serrano. ZE reports no relevant disclosures. AKS has served as a consultant for BMS. MAP has received honoraria from Merck and BMS, a research grant from BMS, and has served on scientific advisory boards for Caladrius, BMS, and Amgen. DBJ is on advisory boards for BMS and Genoptix. JW has grant support from BMS and personal fees from BMS and Merck. AR has received personal fees from Merck, Novartis, Genentech, GlaxoSmithKline, Flexus, Compugen, and Amgen. He has ownership/stock in Kita Pharma.
Figures

Comment in
-
Disparate clinical activity of PD-1 blockade in melanoma subtypes: Know thy enemy!Cancer. 2016 Nov 15;122(21):3263-3266. doi: 10.1002/cncr.30260. Epub 2016 Aug 17. Cancer. 2016. PMID: 27533794 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103:1000–1007. - PubMed
-
- Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22:3016–3023. - PubMed
-
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

