Transient elastography and APRI score: looking at false positives and false negatives. Diagnostic performance and association to fibrosis staging in chronic hepatitis C
- PMID: 27533769
- PMCID: PMC4988482
- DOI: 10.1590/1414-431X20165432
Transient elastography and APRI score: looking at false positives and false negatives. Diagnostic performance and association to fibrosis staging in chronic hepatitis C
Abstract
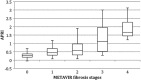
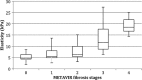
Although long regarded as the gold standard for liver fibrosis staging in chronic hepatitis C (CHC), liver biopsy (LB) implies both the risk of an invasive procedure and significant variability. The aim of this study was to evaluate the diagnostic performance for transient elastography (TE) and aspartate aminotransferase to platelet index (APRI) used alone and in combination compared to liver biopsy and to analyze false positive/negative results. Patients with CHC, and no previous clinical diagnosis of cirrhosis were enrolled to undergo liver biopsy, TE and APRI. A total of 182 adult patients with a median age of 55 years and median body mass index of 26.71 kg/m2 were analyzed. On LB, 56% of patients had significant levels of fibrosis (METAVIR F≥2) and 28% had advanced fibrosis (F3/F4). The strongest performance for both tests was observed for exclusion of advanced fibrosis with good negative predictive values (89 and 86%, respectively). Low necroinflammatory activity on LB was associated with false negative TE. False positives were associated with NASH and smaller LB fragments. Correlation between APRI and Fibroscan for F≥2 was 100% and 84% for F≥3 and remained high in both false negative and false positive instances, correctly identifying F<2 in 71% of cases and F<3 in 78% (and potentially foregoing up to 84% of LB). We concluded that low individual performance indicators could be attributable to limitations of LB. Poorer differentiation of lower levels of fibrosis is a known issue for LB and remains so for noninvasive tests. Good predictability is possible, however, for advanced fibrosis.
Figures

Similar articles
-
Staging liver fibrosis and cirrhosis using non-invasive tests in people with chronic hepatitis B to inform WHO 2024 guidelines: a systematic review and meta-analysis.Lancet Gastroenterol Hepatol. 2025 Apr;10(4):332-349. doi: 10.1016/S2468-1253(24)00437-0. Epub 2025 Feb 18. Lancet Gastroenterol Hepatol. 2025. PMID: 39983746
-
Acoustic radiation force impulse elastography and serum fibrosis markers in chronic hepatitis C.Scand J Gastroenterol. 2014 Aug;49(8):986-92. doi: 10.3109/00365521.2014.909528. Epub 2014 Apr 17. Scand J Gastroenterol. 2014. PMID: 24742130
-
Latent Class Analysis of Noninvasive Methods and Liver Biopsy in Chronic Hepatitis C: An Approach without a Gold Standard.Biomed Res Int. 2017;2017:8252980. doi: 10.1155/2017/8252980. Epub 2017 Sep 13. Biomed Res Int. 2017. PMID: 29057268 Free PMC article.
-
Fibrosis index based on four factors better predicts advanced fibrosis or cirrhosis than aspartate aminotransferase/platelet ratio index in chronic hepatitis C patients.J Formos Med Assoc. 2015 Oct;114(10):923-8. doi: 10.1016/j.jfma.2015.07.004. Epub 2015 Aug 13. J Formos Med Assoc. 2015. PMID: 26279173
-
Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis.Hepatology. 2011 Mar;53(3):726-36. doi: 10.1002/hep.24105. Epub 2011 Feb 11. Hepatology. 2011. PMID: 21319189 Review.
Cited by
-
C-reactive protein to lymphocyte count ratio is a promising novel marker in hepatitis C infection: the clear hep-c study.Rev Assoc Med Bras (1992). 2022 Jun 24;68(6):838-841. doi: 10.1590/1806-9282.20220236. eCollection 2022. Rev Assoc Med Bras (1992). 2022. PMID: 35766701 Free PMC article.
-
Hyperuricemia Is Associated with Significant Liver Fibrosis in Subjects with Nonalcoholic Fatty Liver Disease, but Not in Subjects without It.J Clin Med. 2022 Mar 7;11(5):1445. doi: 10.3390/jcm11051445. J Clin Med. 2022. PMID: 35268536 Free PMC article.
-
All-oral direct antiviral treatment for hepatitis C chronic infection in a real-life cohort: The role of cirrhosis and comorbidities in treatment response.PLoS One. 2018 Jul 10;13(7):e0199941. doi: 10.1371/journal.pone.0199941. eCollection 2018. PLoS One. 2018. PMID: 29990371 Free PMC article.
-
Use of aspartate aminotransferase to platelet ratio to reduce the need for FibroScan in the evaluation of liver fibrosis.World J Hepatol. 2017 Jun 18;9(17):791-796. doi: 10.4254/wjh.v9.i17.791. World J Hepatol. 2017. PMID: 28660013 Free PMC article.
-
Underestimation of Liver Fibrosis Using Vibration-Controlled Transient Elastography on Cirrhosis. Are There Predictors?Can Liver J. 2025 Feb 25;8(1):18-28. doi: 10.3138/canlivj-2024-0038. eCollection 2025 Feb. Can Liver J. 2025. PMID: 40678663 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

