Total synthesis of fellutamides, lipopeptide proteasome inhibitors. More sustainable peptide bond formation
- PMID: 27533920
- PMCID: PMC5303010
- DOI: 10.1039/c6ob01233g
Total synthesis of fellutamides, lipopeptide proteasome inhibitors. More sustainable peptide bond formation
Erratum in
-
Correction: Total synthesis of fellutamides, lipopeptide proteasome inhibitors. More sustainable peptide bond formation.Org Biomol Chem. 2016 Sep 26;14(38):9159. doi: 10.1039/c6ob90139e. Org Biomol Chem. 2016. PMID: 27714303
Abstract
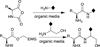
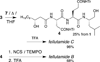
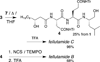
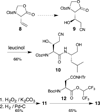
Solution-phase syntheses of three bioactive natural products of mixed polypeptide-polyketide biogenesis, fellutamides A, B, and C, have been achieved. Three peptide bonds are generated without the use of coupling reagents in each synthesis of the fellutamides, which act against proteasomes.
Figures

References
-
- Shigemori H, Wakuri S, Yazawa K, Nakamura T, Sasaki T, Kobayashi J. Tetrahedron. 1991;47:8529–8534.
- Yamaguchi K, Tsuji T, Wakuri S, Yazawa K, Kondo K, Shigemori H, Kobayashi J. Biosci. Biotechnol. Biochem. 1993;57:195–199. - PubMed
- Xu D, Ondeyka J, Harris GH, Zink D, Kahn JN, Wang H, Bills G, Platas G, Wang W, Szewczak AA, Liberator P, Roemer T, Singh SB. J. Nat. Prod. 2011;74:1721–1730. - PubMed
- Lee YM, Dang TH, Hong J, Lee CO, Bae KS, Kim DK, Jung JH. Bull. Korean Chem. Soc. 2010;31:205–208.
- Lee YM, Dang HT, Li J, Zhang P, Hong J, Lee C-O, Jung JH. Bull. Korean Chem. Soc. 2011;32:3817–3820.
-
- Schneekloth JS, Jr, Sanders JL, Hines J, Crews CM. Bioorg. Med. Chem. Lett. 2006;16:3855–3858. - PMC - PubMed
- Dang HT, Xiao B, Lee YM, Hong J, Lee C-O, Choi JS, Jung JH. Bull. Korean Chem. Soc. 2012;33:2777–2780.
- Giltrap AM, Cergol KM, Pang A, Britton WJ, Payne RJ. Mar. Drugs. 2013;11:2382–2397. - PMC - PubMed
-
- Pirrung MC, Biswas G, Ibarra-Rivera T. Org. Lett. 2010;12:2402–245. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

