Knockdown of GSK3β increases basal autophagy and AMPK signalling in nutrient-laden human aortic endothelial cells
- PMID: 27534430
- PMCID: PMC5025810
- DOI: 10.1042/BSR20160174
Knockdown of GSK3β increases basal autophagy and AMPK signalling in nutrient-laden human aortic endothelial cells
Abstract
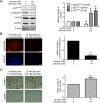
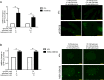
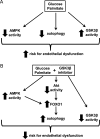
High concentrations of glucose and palmitate increase endothelial cell inflammation and apoptosis, events that often precede atherogenesis. They may do so by decreasing basal autophagy and AMP-activated protein kinase (AMPK) activity, although the mechanisms by which this occurs are not clear. Decreased function of the lysosome, an organelle required for autophagy and AMPK, have been associated with hyperactivity of glycogen synthase kinase 3β (GSK3β). To determine whether GSK3β affects nutrient-induced changes in autophagy and AMPK activity, we used a primary human aortic endothelial cell (HAEC) model of type 2 diabetes that we had previously characterized with impaired AMPK activity and autophagy [Weikel et al. (2015) Am. J. Phys. Cell Physiol. 308: , C249-C263]. Presently, we found that incubation of HAECs with excess nutrients (25 mM glucose and 0.4 mM palmitate) increased GSK3β activity and impaired lysosome acidification. Suppression of GSK3β in these cells by treatment with a chemical inhibitor or overexpression of kinase-dead GSK3β attenuated these lysosomal changes. Under control and excess nutrient conditions, knockdown of GSK3β increased autophagosome formation, forkhead box protein O1 (FOXO1) activity and AMPK signalling and decreased Akt signalling. Similar changes in autophagy, AMPK and Akt signalling were observed in aortas from mice treated with the GSK3β inhibitor CHIR 99021. Thus, increasing basal autophagy and AMPK activity by inhibiting GSK3β may be an effective strategy in the setting of hyperglycaemia and dyslipidaemia for restoring endothelial cell health and reducing atherogenesis.
Keywords: AMP-activated protein kinase (AMPK); autophagy; endothelium; forkhead box protein O1 (FOXO1); glycogen synthase kinase 3β (GSK3β).
© 2016 The Author(s).
Figures





Similar articles
-
Ampelopsin protects endothelial cells from hyperglycemia-induced oxidative damage by inducing autophagy via the AMPK signaling pathway.Biofactors. 2015 Nov-Dec;41(6):463-75. doi: 10.1002/biof.1248. Epub 2015 Dec 8. Biofactors. 2015. PMID: 26644014
-
Glucose and palmitate uncouple AMPK from autophagy in human aortic endothelial cells.Am J Physiol Cell Physiol. 2015 Feb 1;308(3):C249-63. doi: 10.1152/ajpcell.00265.2014. Epub 2014 Oct 29. Am J Physiol Cell Physiol. 2015. PMID: 25354528 Free PMC article.
-
Glycogen synthase kinase 3β promotes liver innate immune activation by restraining AMP-activated protein kinase activation.J Hepatol. 2018 Jul;69(1):99-109. doi: 10.1016/j.jhep.2018.01.036. Epub 2018 Feb 13. J Hepatol. 2018. PMID: 29452207 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Metabolic aspects of cardiovascular diseases: Is FoxO1 a player or a target?Int J Biochem Cell Biol. 2020 Jan;118:105659. doi: 10.1016/j.biocel.2019.105659. Epub 2019 Nov 22. Int J Biochem Cell Biol. 2020. PMID: 31765819 Review.
Cited by
-
Spinosin and 6'''‑Feruloylspinosin protect the heart against acute myocardial ischemia and reperfusion in rats.Mol Med Rep. 2019 Nov;20(5):4253-4261. doi: 10.3892/mmr.2019.10686. Epub 2019 Sep 16. Mol Med Rep. 2019. PMID: 31545438 Free PMC article.
-
Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors.Curr Neurovasc Res. 2018;15(1):81-91. doi: 10.2174/1567202615666180319151244. Curr Neurovasc Res. 2018. PMID: 29557749 Free PMC article. Review.
-
Forkhead Transcription Factors: Formulating a FOXO Target for Cognitive Loss.Curr Neurovasc Res. 2017;14(4):415-420. doi: 10.2174/1567202614666171116102911. Curr Neurovasc Res. 2017. PMID: 29149835 Free PMC article. Review.
-
Ataxia telangiectasia mutated kinase deficiency impairs the autophagic response early during myocardial infarction.Am J Physiol Heart Circ Physiol. 2018 Jul 1;315(1):H48-H57. doi: 10.1152/ajpheart.00042.2018. Epub 2018 Apr 13. Am J Physiol Heart Circ Physiol. 2018. PMID: 29652546 Free PMC article.
-
The role of GSK3 and its reversal with GSK3 antagonism in everolimus resistance.Endocr Relat Cancer. 2018 Oct;25(10):893-908. doi: 10.1530/ERC-18-0159. Epub 2018 Jun 12. Endocr Relat Cancer. 2018. PMID: 29895527 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

