Upregulations of CRH and CRHR1 in the Epileptogenic Tissues of Patients with Intractable Infantile Spasms
- PMID: 27534449
- PMCID: PMC6492682
- DOI: 10.1111/cns.12598
Upregulations of CRH and CRHR1 in the Epileptogenic Tissues of Patients with Intractable Infantile Spasms
Abstract
Aim: Infantile spasms (IS) are an age-specific epileptic syndrome with specific clinical symptom and electroencephalogram (EEG) features, lacking treatment options, and a poor prognosis. Excessive endogenous corticotropin-releasing hormone (CRH) in infant brain might result in IS. However, the data from human IS are limited. In our study, we investigated the expressions of CRH and its receptor type 1 (CRHR1) in surgical tissues from patients with IS and autopsy controls.
Methods: Specimens surgically removed from 17 patients with IS, and six autopsy controls were included in the study. Real-time PCR, Western blotting, and immunostaining were used to detect the expressions of mRNA, protein expression, and distribution. The correlation between variates was analyzed by Spearman rank correlation.
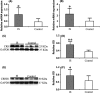
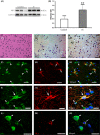
Results: The expressions of CRH and CRHR1 were significantly upregulated in the epileptogenic tissues of IS patients compared with the control group. CRH was distributed mainly in neurons, while CRHR1 was distributed in neurons, astrocytes, and microglia. The expression levels of CRH and CRHR1 were positively correlated with the frequency of epileptic spasms. Moreover, the expression of protein kinase C (PKC), which was an important downstream factor of CRHR1, was significantly upregulated in the epileptogenic tissues of patients with IS and was positively correlated with the CRHR1 expression levels and the frequency of epileptic spasms.
Conclusion: These results suggest that the CRH signal transduction pathway might participate in the epileptogenesis of IS, supporting the hypothesis that CRH is related to the pathogenesis of IS.
Keywords: Corticotrophin-releasing hormone receptor 1 (CRHR1); Corticotropin-releasing hormone (CRH); Infantile spasms (IS); Protein Kinase C (PKC).
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Differential expression of CRH, UCN, CRHR1 and CRHR2 in eutopic and ectopic endometrium of women with endometriosis.PLoS One. 2013 Apr 24;8(4):e62313. doi: 10.1371/journal.pone.0062313. Print 2013. PLoS One. 2013. PMID: 23638035 Free PMC article.
-
Fish oil enhances intestinal barrier function and inhibits corticotropin-releasing hormone/corticotropin-releasing hormone receptor 1 signalling pathway in weaned pigs after lipopolysaccharide challenge.Br J Nutr. 2016 Jun;115(11):1947-57. doi: 10.1017/S0007114516001100. Epub 2016 Apr 15. Br J Nutr. 2016. PMID: 27080003
-
A single episode of restraint stress regulates central corticotrophin- releasing hormone receptor expression and binding in specific areas of the mouse brain.J Neuroendocrinol. 2009 May;21(5):473-80. doi: 10.1111/j.1365-2826.2009.01865.x. J Neuroendocrinol. 2009. PMID: 19302188
-
Corticotropin releasing hormone receptors: two decades later.Peptides. 2004 Mar;25(3):319-29. doi: 10.1016/j.peptides.2004.02.002. Peptides. 2004. PMID: 15134857 Review.
-
Milestones in CRH Research.Curr Mol Pharmacol. 2017;10(4):259-263. doi: 10.2174/1874467210666170109165219. Curr Mol Pharmacol. 2017. PMID: 28071586 Review.
Cited by
-
Hypothalamic-Pituitary-Adrenal Axis and Epilepsy.J Clin Neurol. 2024 Mar;20(2):131-139. doi: 10.3988/jcn.2023.0308. Epub 2024 Feb 5. J Clin Neurol. 2024. PMID: 38330420 Free PMC article. Review.
-
Elevated Expression of TRPC4 in Cortical Lesions of Focal Cortical Dysplasia II and Tuberous Sclerosis Complex.J Mol Neurosci. 2017 Jun;62(2):222-231. doi: 10.1007/s12031-017-0923-z. Epub 2017 Apr 28. J Mol Neurosci. 2017. PMID: 28455787
-
Downregulated GPR30 expression in the epileptogenic foci of female patients with focal cortical dysplasia type IIb and tuberous sclerosis complex is correlated with 18 F-FDG PET-CT values.Brain Pathol. 2021 Mar;31(2):346-364. doi: 10.1111/bpa.12925. Epub 2021 Feb 10. Brain Pathol. 2021. PMID: 33314369 Free PMC article.
-
Investigation of the association between imbalance of the intestinal flora and infantile spasms: a pilot case-control study.Transl Pediatr. 2021 Apr;10(4):819-833. doi: 10.21037/tp-20-384. Transl Pediatr. 2021. PMID: 34012831 Free PMC article.
-
Aetiopathogenesis of infantile epileptic spasms syndrome and mechanisms of action of adrenocorticotrophin hormone/corticosteroids in children: A scoping review.Dev Med Child Neurol. 2025 Aug;67(8):1004-1025. doi: 10.1111/dmcn.16273. Epub 2025 Feb 28. Dev Med Child Neurol. 2025. PMID: 40019827 Free PMC article.
References
-
- Sorel L, Dusaucy‐Bauloye A. Findings in 21 cases of Gibbs’ hypsarrhythmia; spectacular effectiveness of ACTH. Acta Neurol Psychiatr Belg 1958;58:130–141. - PubMed
-
- Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res 1983;278:332–336. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

