CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson's disease
- PMID: 27534533
- PMCID: PMC4892852
- DOI: 10.1038/emm.2015.100
CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson's disease
Abstract
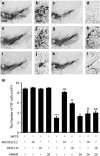
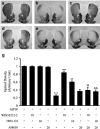
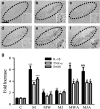
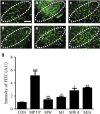
The cannabinoid (CB2) receptor type 2 has been proposed to prevent the degeneration of dopamine neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. However, the mechanisms underlying CB2 receptor-mediated neuroprotection in MPTP mice have not been elucidated. The mechanisms underlying CB2 receptor-mediated neuroprotection of dopamine neurons in the substantia nigra (SN) were evaluated in the MPTP mouse model of Parkinson's disease (PD) by immunohistochemical staining (tyrosine hydroxylase, macrophage Ag complex-1, glial fibrillary acidic protein, myeloperoxidase (MPO), and CD3 and CD68), real-time PCR and a fluorescein isothiocyanate-labeled albumin assay. Treatment with the selective CB2 receptor agonist JWH-133 (10 μg kg(-1), intraperitoneal (i.p.)) prevented MPTP-induced degeneration of dopamine neurons in the SN and of their fibers in the striatum. This JWH-133-mediated neuroprotection was associated with the suppression of blood-brain barrier (BBB) damage, astroglial MPO expression, infiltration of peripheral immune cells and production of inducible nitric oxide synthase, proinflammatory cytokines and chemokines by activated microglia. The effects of JWH-133 were mimicked by the non-selective cannabinoid receptor WIN55,212 (10 μg kg(-1), i.p.). The observed neuroprotection and inhibition of glial-mediated neurotoxic events were reversed upon treatment with the selective CB2 receptor antagonist AM630, confirming the involvement of the CB2 receptor. Our results suggest that targeting the cannabinoid system may be beneficial for the treatment of neurodegenerative diseases, such as PD, that are associated with glial activation, BBB disruption and peripheral immune cell infiltration.
Figures



References
-
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 2007; 8: 57–69. - PubMed
-
- Chung YC, Ko HW, Bok E, Park ES, Huh SH, Nam JH et al. The role of neuroinflammation on the pathogenesis of Parkinson's disease. BMB Rep 2010; 43: 225–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

