Three nested randomized controlled trials of peer-only or multiple stakeholder group feedback within Delphi surveys during core outcome and information set development
- PMID: 27534622
- PMCID: PMC4989325
- DOI: 10.1186/s13063-016-1479-x
Three nested randomized controlled trials of peer-only or multiple stakeholder group feedback within Delphi surveys during core outcome and information set development
Abstract
Background: Methods for developing a core outcome or information set require involvement of key stakeholders to prioritise many items and achieve agreement as to the core set. The Delphi technique requires participants to rate the importance of items in sequential questionnaires (or rounds) with feedback provided in each subsequent round such that participants are able to consider the views of others. This study examines the impact of receiving feedback from different stakeholder groups, on the subsequent rating of items and the level of agreement between stakeholders.
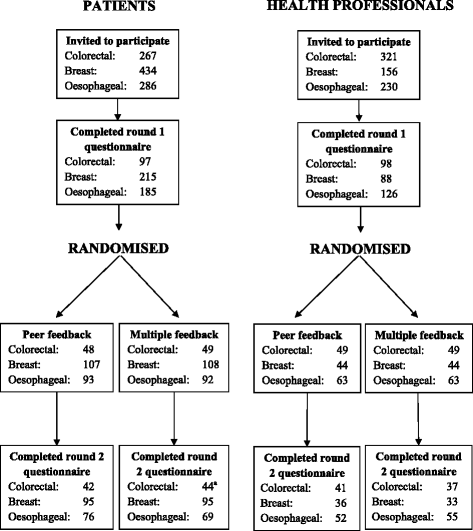
Methods: Randomized controlled trials were nested within the development of three core sets each including a Delphi process with two rounds of questionnaires, completed by patients and health professionals. Participants rated items from 1 (not essential) to 9 (absolutely essential). For round 2, participants were randomized to receive feedback from their peer stakeholder group only (peer) or both stakeholder groups separately (multiple). Decisions as to which items to retain following each round were determined by pre-specified criteria.
Results: Whilst type of feedback did not impact on the percentage of items for which a participant subsequently changed their rating, or the magnitude of change, it did impact on items retained at the end of round 2. Each core set contained discordant items retained by one feedback group but not the other (3-22 % discordant items). Consensus between patients and professionals in items to retain was greater amongst those receiving multiple group feedback in each core set (65-82 % agreement for peer-only feedback versus 74-94 % for multiple feedback). In addition, differences in round 2 scores were smaller between stakeholder groups receiving multiple feedback than between those receiving peer group feedback only. Variability in item scores across stakeholders was reduced following any feedback but this reduction was consistently greater amongst the multiple feedback group.
Conclusions: In the development of a core outcome or information set, providing feedback within Delphi questionnaires from all stakeholder groups separately may influence the final core set and improve consensus between the groups. Further work is needed to better understand how participants rate and re-rate items within a Delphi process.
Trial registration: The three randomized controlled trials reported here were each nested within the development of a core information or outcome set to investigate processes in core outcome and information set development. Outcomes were not health-related and therefore trial registration was not applicable.
Keywords: Consensus; Core information set; Core outcome set; Delphi; Feedback.
Figures



Similar articles
-
Impact of question order on prioritisation of outcomes in the development of a core outcome set: a randomised controlled trial.Trials. 2018 Jan 25;19(1):66. doi: 10.1186/s13063-017-2405-6. Trials. 2018. PMID: 29370827 Free PMC article. Clinical Trial.
-
A randomized trial comparing three Delphi feedback strategies found no evidence of a difference in a setting with high initial agreement.J Clin Epidemiol. 2018 Jan;93:1-8. doi: 10.1016/j.jclinepi.2017.09.024. Epub 2017 Oct 7. J Clin Epidemiol. 2018. PMID: 29017811 Clinical Trial.
-
Multi-Round versus Real-Time Delphi survey approach for achieving consensus in the COHESION core outcome set: a randomised trial.Trials. 2023 Jul 19;24(1):461. doi: 10.1186/s13063-023-07388-9. Trials. 2023. PMID: 37468987 Free PMC article. Clinical Trial.
-
Core Outcome Set for Actinic Keratosis Clinical Trials.JAMA Dermatol. 2020 Mar 1;156(3):326-333. doi: 10.1001/jamadermatol.2019.4212. JAMA Dermatol. 2020. PMID: 31939999
-
TREatment of ATopic eczema (TREAT) Registry Taskforce: protocol for an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema registries.Trials. 2017 Feb 27;18(1):87. doi: 10.1186/s13063-016-1765-7. Trials. 2017. PMID: 28241851 Free PMC article.
Cited by
-
Comparison of different rating scales for the use in Delphi studies: different scales lead to different consensus and show different test-retest reliability.BMC Med Res Methodol. 2020 Feb 10;20(1):28. doi: 10.1186/s12874-020-0912-8. BMC Med Res Methodol. 2020. PMID: 32041541 Free PMC article.
-
The CORE-KDT study: a mixed methods protocol to establish core outcomes for refractory childhood epilepsy treated with ketogenic diet therapy.Trials. 2022 Aug 17;23(1):675. doi: 10.1186/s13063-022-06629-7. Trials. 2022. PMID: 35978413 Free PMC article.
-
Development of the CORE-Kids core set of outcome domains for studies of childhood limb fractures.Bone Joint J. 2021 Dec;103-B(12):1821-1830. doi: 10.1302/0301-620X.103B.BJJ-2020-2321.R2. Epub 2021 Aug 19. Bone Joint J. 2021. PMID: 34412506 Free PMC article.
-
Core Outcome Set-STAndards for Reporting: The COS-STAR Statement.PLoS Med. 2016 Oct 18;13(10):e1002148. doi: 10.1371/journal.pmed.1002148. eCollection 2016 Oct. PLoS Med. 2016. PMID: 27755541 Free PMC article.
-
The COMET Handbook: version 1.0.Trials. 2017 Jun 20;18(Suppl 3):280. doi: 10.1186/s13063-017-1978-4. Trials. 2017. PMID: 28681707 Free PMC article. Review.
References
-
- COMET (Core Outcome Measures in Effectiveness Trials) Initiative. http://www.comet-initiative.org/. Accessed 09 Apr 2015.
-
- Blazeby JM, Macefield RC, Blencowe NS, Jacobs M, McNair AG, Sprangers M, Brookes ST. A core information set for surgery for oesophageal cancer. Br J Surg. 2015;102(8):936–43. - PubMed
-
- Beauchamp TL, Childress JF. Principles of biomedical ethics. Oxford: Oxford University Press; 2001.
-
- Main B, Davies L, McNair AG, Blazeby JM. Bringing informed consent back to patients. BMJ Blogs 2014. http://blogs.bmj.com/bmj/2014/08/05/barry-main-et-al-bringing-informed-c.... Accessed 09 Apr 2015.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

