Reassessing wanting and liking in the study of mesolimbic influence on food intake
- PMID: 27534877
- PMCID: PMC5130579
- DOI: 10.1152/ajpregu.00234.2016
Reassessing wanting and liking in the study of mesolimbic influence on food intake
Abstract
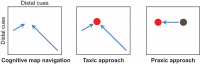
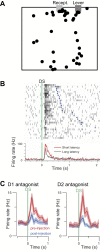
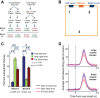
Humans and animals such as rats and mice tend to overconsume calorie-dense foods, a phenomenon that likely contributes to obesity. One often-advanced explanation for why we preferentially consume sweet and fatty foods is that they are more "rewarding" than low-calorie foods. "Reward" has been subdivided into three interdependent psychological processes: hedonia (liking a food), reinforcement (formation of associations among stimuli, actions, and/or the food), and motivation (wanting the food). Research into these processes has focused on the mesolimbic system, which comprises both dopamine neurons in the ventral tegmental area and neurons in their major projection target, the nucleus accumbens. The mesolimbic system and closely connected structures are commonly referred to as the brain's "reward circuit." Implicit in this title is the assumption that "rewarding" experiences are generally the result of activity in this circuit. In this review, I argue that food intake and the preference for calorie-dense foods can be explained without reference to subjective emotions. Furthermore, the contribution of mesolimbic dopamine to food intake and preference may not be a general one of promoting or coordinating behaviors that result in the most reward or caloric intake but may instead be limited to the facilitation of a specific form of neural computation that results in conditioned approach behavior. Studies on the neural mechanisms of caloric intake regulation must address how sensory information about calorie intake affects not just the mesolimbic system but also many other forms of computation that govern other types of food-seeking and food-oriented behaviors.
Keywords: liking; reward; wanting.
Copyright © 2016 the American Physiological Society.
Figures
References
-
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116: 3229–3239, 2006. doi:10.1172/JCI29867. - DOI - PMC - PubMed
-
- Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol 34B: 77–98, 1982. doi:10.1080/14640748208400878. - DOI
-
- Adams CD, Dickinson A. Instrumental responding following reinforcer devaluation. Q J Exp Psychol 33: 109–121, 1981. doi:10.1080/14640748108400816. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

