Catalytic iron and acute kidney injury
- PMID: 27534995
- PMCID: PMC5130458
- DOI: 10.1152/ajprenal.00388.2016
Catalytic iron and acute kidney injury
Abstract
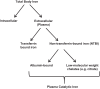
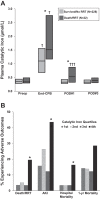
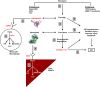
Acute kidney injury (AKI) is a common and often devastating condition among hospitalized patients and is associated with markedly increased hospital length of stay, mortality, and cost. The pathogenesis of AKI is complex, but animal models support an important role for catalytic iron in causing AKI. Catalytic iron, also known as labile iron, is a transitional pool of non-transferrin-bound iron that is readily available to participate in redox cycling. Initial findings related to catalytic iron and animal models of kidney injury have only recently been extended to human AKI. In this review, we discuss the role of catalytic iron in human AKI, focusing on recent translational studies in humans, assay considerations, and potential therapeutic targets for future interventional studies.
Keywords: AKI; HO-1; ferritin; labile iron; non-transferrin-bound iron.
Copyright © 2016 the American Physiological Society.
Figures
Similar articles
-
Iron Chelation as a Potential Therapeutic Strategy for AKI Prevention.J Am Soc Nephrol. 2019 Nov;30(11):2060-2071. doi: 10.1681/ASN.2019060595. Epub 2019 Sep 25. J Am Soc Nephrol. 2019. PMID: 31554656 Free PMC article. Review.
-
Iron, ferroptosis, and new insights for prevention in acute kidney injury.Adv Med Sci. 2020 Sep;65(2):361-370. doi: 10.1016/j.advms.2020.06.004. Epub 2020 Jun 25. Adv Med Sci. 2020. PMID: 32592957 Review.
-
Iron Homeostasis and Ferritin in Sepsis-Associated Kidney Injury.Nephron. 2020;144(12):616-620. doi: 10.1159/000508857. Epub 2020 Jul 21. Nephron. 2020. PMID: 32694248 Free PMC article. Review.
-
Cellular and Molecular Mechanisms of AKI.J Am Soc Nephrol. 2016 May;27(5):1288-99. doi: 10.1681/ASN.2015070740. Epub 2016 Feb 9. J Am Soc Nephrol. 2016. PMID: 26860342 Free PMC article. Review.
-
Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis.Nephrology (Carlton). 2015 Jul;20(7):485-91. doi: 10.1111/nep.12439. Nephrology (Carlton). 2015. PMID: 25726708
Cited by
-
Anemia of Inflammation with An Emphasis on Chronic Kidney Disease.Nutrients. 2019 Oct 11;11(10):2424. doi: 10.3390/nu11102424. Nutrients. 2019. PMID: 31614529 Free PMC article. Review.
-
Cardiac Surgery Associated AKI Prevention Strategies and Medical Treatment for CSA-AKI.J Clin Med. 2021 Nov 14;10(22):5285. doi: 10.3390/jcm10225285. J Clin Med. 2021. PMID: 34830567 Free PMC article.
-
Ferritin in Kidney and Vascular Related Diseases: Novel Roles for an Old Player.Pharmaceuticals (Basel). 2019 Jun 21;12(2):96. doi: 10.3390/ph12020096. Pharmaceuticals (Basel). 2019. PMID: 31234273 Free PMC article. Review.
-
Acute kidney injury: emerging pharmacotherapies in current clinical trials.Pediatr Nephrol. 2018 May;33(5):779-787. doi: 10.1007/s00467-017-3695-3. Epub 2017 Jun 10. Pediatr Nephrol. 2018. PMID: 28601936 Free PMC article. Review.
-
Prevention of Cardiac Surgery-Associated Acute Kidney Injury: A Review of Current Strategies.Anesthesiol Clin. 2019 Dec;37(4):729-749. doi: 10.1016/j.anclin.2019.08.007. Epub 2019 Sep 21. Anesthesiol Clin. 2019. PMID: 31677688 Free PMC article. Review.
References
-
- Baliga R, Zhang Z, Baliga M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 49: 362–369, 1996. - PubMed
-
- Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int 53: 394–401, 1998. - PubMed
-
- Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 267: 18148–18153, 1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

