The Q-rich/PST domain of the AHR regulates both ligand-induced nuclear transport and nucleocytoplasmic shuttling
- PMID: 27535013
- PMCID: PMC4989234
- DOI: 10.1038/srep32009
The Q-rich/PST domain of the AHR regulates both ligand-induced nuclear transport and nucleocytoplasmic shuttling
Abstract
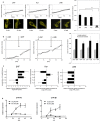
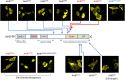
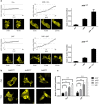
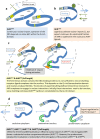
The aryl hydrocarbon receptor (AHR) shuttles continuously between cytoplasm and nucleus, unless ligand-binding triggers association with the AHR nuclear translocator (ARNT) and subsequent binding to cognate DNA motifs. We have now identified Val 647 as mandatory residue for export from the nucleus and AHR-function. This residue prevents inactivation of the receptor as a consequence of nuclear sequestration via constitutive import. Concomitantly mutants lacking this residue are exclusively localised in the nucleus. Although ligands accelerate nuclear import transiently, stable nuclear transition depends on a motif adjacent to Val 647 that comprises residues 650-661. Together, this defined region within the Q-rich domain regulates intracellular trafficking of the AHR in context of both nucleocytoplasmic shuttling and receptor activation. Nuclear export therefore depends on the previously characterised N-terminal NES and the newly identified motif that includes V647. Nucleocytoplasmic distribution of full-length human AHR is further affected by a section of the PST domain that shows sequence similarities with nuclear export signals. In concert, these motifs maintain a predominant cytoplasmic compartmentalisation, receptive for ligand binding.
Figures

Similar articles
-
The role of DNA-binding and ARNT dimerization on the nucleo-cytoplasmic translocation of the aryl hydrocarbon receptor.Sci Rep. 2021 Sep 14;11(1):18194. doi: 10.1038/s41598-021-97507-w. Sci Rep. 2021. PMID: 34521881 Free PMC article.
-
Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor.J Biochem. 2000 Mar;127(3):503-9. doi: 10.1093/oxfordjournals.jbchem.a022633. J Biochem. 2000. PMID: 10731723
-
Nuclear localization and export signals of the human aryl hydrocarbon receptor.J Biol Chem. 1998 Jan 30;273(5):2895-904. doi: 10.1074/jbc.273.5.2895. J Biol Chem. 1998. PMID: 9446600
-
Subcellular Localization Signals of bHLH-PAS Proteins: Their Significance, Current State of Knowledge and Future Perspectives.Int J Mol Sci. 2019 Sep 24;20(19):4746. doi: 10.3390/ijms20194746. Int J Mol Sci. 2019. PMID: 31554340 Free PMC article. Review.
-
Nucleocytoplasmic shuttling of transcription factors.Cell Mol Life Sci. 2000 Aug;57(8-9):1193-206. doi: 10.1007/pl00000759. Cell Mol Life Sci. 2000. PMID: 11028912 Free PMC article. Review.
Cited by
-
Hypothesis: Emerging Roles for Aryl Hydrocarbon Receptor in Orchestrating CoV-2-Related Inflammation.Cells. 2022 Feb 13;11(4):648. doi: 10.3390/cells11040648. Cells. 2022. PMID: 35203299 Free PMC article. Review.
-
The Ah Receptor: Adaptive Metabolism, Ligand Diversity, and the Xenokine Model.Chem Res Toxicol. 2020 Apr 20;33(4):860-879. doi: 10.1021/acs.chemrestox.9b00476. Epub 2020 Apr 7. Chem Res Toxicol. 2020. PMID: 32259433 Free PMC article. Review.
-
The role of DNA-binding and ARNT dimerization on the nucleo-cytoplasmic translocation of the aryl hydrocarbon receptor.Sci Rep. 2021 Sep 14;11(1):18194. doi: 10.1038/s41598-021-97507-w. Sci Rep. 2021. PMID: 34521881 Free PMC article.
-
The IDO inhibitor 1-methyl tryptophan activates the aryl hydrocarbon receptor response in mesenchymal stromal cells.Oncotarget. 2017 Aug 10;8(54):91914-91927. doi: 10.18632/oncotarget.20166. eCollection 2017 Nov 3. Oncotarget. 2017. PMID: 29190885 Free PMC article.
-
Gene and Protein Expression in Subjects With a Nystagmus-Associated AHR Mutation.Front Genet. 2020 Sep 24;11:582796. doi: 10.3389/fgene.2020.582796. eCollection 2020. Front Genet. 2020. PMID: 33193710 Free PMC article.
References
-
- Gu Y. Z., Hogenesch J. B. & Bradfield C. A. The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. A 40, 519–561 (2000). - PubMed
-
- Bock K. W. & Kohle C. The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. Biol. Chem. 390, 1225–1235 (2009). - PubMed
-
- Poland A., Clover E., Kende A. S., DeCamp M. & Giandomenico C. M. 3,4,3′,4′-Tetrachloro azoxybenzene and azobenzene: potent inducers of aryl hydrocarbon hydroxylase. Science 194, 627–630 (1976). - PubMed
-
- Henkler F., Stolpmann K. & Luch A. Exposure to polycyclic aromatic hydrocarbons: bulky DNA adducts and cellular responses. EXS 101, 107–131 (2012). - PubMed
-
- Luch A. Nature and nurture — lessons from chemical carcinogenesis. Nat. Rev. Cancer 5, 113–125 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

