The inferior olive is essential for long-term maintenance of a simple motor skill
- PMID: 27535367
- PMCID: PMC5144694
- DOI: 10.1152/jn.00085.2016
The inferior olive is essential for long-term maintenance of a simple motor skill
Abstract
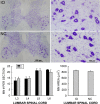
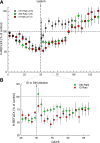
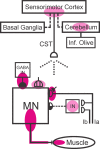
The inferior olive (IO) is essential for operant down-conditioning of the rat soleus H-reflex, a simple motor skill. To evaluate the role of the IO in long-term maintenance of this skill, the H-reflex was down-conditioned over 50 days, the IO was chemically ablated, and down-conditioning continued for up to 102 more days. H-reflex size just before IO ablation averaged 62(±2 SE)% of its initial value (P < 0.001 vs. initial). After IO ablation, H-reflex size rose to 75-80% over ∼10 days, remained there for ∼30 days, rose over 10 days to above its initial value, and averaged 140(±14)% for the final 10 days of study (P < 0.01 vs. initial). This two-stage loss of down-conditioning maintenance correlated with IO neuronal loss (r = 0.75, P < 0.01) and was similar to the loss of down-conditioning that follows ablation of the cerebellar output nuclei dentate and interpositus. In control (i.e., unconditioned) rats, IO ablation has no long-term effect on H-reflex size. These results indicate that the IO is essential for long-term maintenance of a down-conditioned H-reflex. With previous data, they support the hypothesis that IO and cortical inputs to cerebellum combine to produce cerebellar plasticity that produces sensorimotor cortex plasticity that produces spinal cord plasticity that produces the smaller H-reflex. H-reflex down-conditioning appears to depend on a hierarchy of plasticity that may be guided by the IO and begin in the cerebellum. Similar hierarchies may underlie other motor learning.
Keywords: H-reflex; cerebellum; learning; memory; operant conditioning; plasticity; sensorimotor cortex; spinal cord.
Figures


Similar articles
-
The cerebellum in maintenance of a motor skill: a hierarchy of brain and spinal cord plasticity underlies H-reflex conditioning.Learn Mem. 2006 Mar-Apr;13(2):208-15. doi: 10.1101/lm.92706. Learn Mem. 2006. PMID: 16585796 Free PMC article.
-
Ablation of the inferior olive prevents H-reflex down-conditioning in rats.J Neurophysiol. 2016 Mar;115(3):1630-6. doi: 10.1152/jn.01069.2015. Epub 2016 Jan 20. J Neurophysiol. 2016. PMID: 26792888 Free PMC article.
-
Sensorimotor cortex ablation prevents H-reflex up-conditioning and causes a paradoxical response to down-conditioning in rats.J Neurophysiol. 2006 Jul;96(1):119-27. doi: 10.1152/jn.01271.2005. Epub 2006 Apr 5. J Neurophysiol. 2006. PMID: 16598062
-
The simplest motor skill: mechanisms and applications of reflex operant conditioning.Exerc Sport Sci Rev. 2014 Apr;42(2):82-90. doi: 10.1249/JES.0000000000000010. Exerc Sport Sci Rev. 2014. PMID: 24508738 Free PMC article. Review.
-
Restoring walking after spinal cord injury: operant conditioning of spinal reflexes can help.Neuroscientist. 2015 Apr;21(2):203-15. doi: 10.1177/1073858414527541. Epub 2014 Mar 17. Neuroscientist. 2015. PMID: 24636954 Free PMC article. Review.
Cited by
-
The negotiated equilibrium model of spinal cord function.J Physiol. 2018 Aug;596(16):3469-3491. doi: 10.1113/JP275532. Epub 2018 Jul 10. J Physiol. 2018. PMID: 29663410 Free PMC article. Review.
-
Suprapontine Structures Modulate Brainstem and Spinal Networks.Cell Mol Neurobiol. 2023 Aug;43(6):2831-2856. doi: 10.1007/s10571-023-01321-z. Epub 2023 Feb 2. Cell Mol Neurobiol. 2023. PMID: 36732488 Free PMC article.
-
Acquisition, Maintenance, and Therapeutic Use of a Simple Motor Skill.Curr Opin Behav Sci. 2018 Apr;20:138-144. doi: 10.1016/j.cobeha.2017.12.021. Epub 2018 Feb 3. Curr Opin Behav Sci. 2018. PMID: 30480059 Free PMC article.
-
Roles, molecular mechanisms, and signaling pathways of TMEMs in neurological diseases.Am J Transl Res. 2021 Dec 15;13(12):13273-13297. eCollection 2021. Am J Transl Res. 2021. PMID: 35035675 Free PMC article. Review.
-
Operant down-conditioning of the soleus H-reflex in people after stroke.Front Rehabil Sci. 2022 Jul 22;3:859724. doi: 10.3389/fresc.2022.859724. eCollection 2022. Front Rehabil Sci. 2022. PMID: 36188979 Free PMC article.
References
-
- Andre P, Arrogi P. Hipnic modulation of cerebellar information processing: implications for the cerebro-cerebellar dialogue. Cerebellum 2: 84–95, 2003. - PubMed
-
- Aoki H, Sugihara I. Morphology of single olivocerebellar axons in the denervation-reinnervation model produced by subtotal lesion of the rat inferior olive. Brain Res 1449: 24–37, 2012. - PubMed
-
- Balaban CD. Central neurotoxic effects of intraperitoneal administered 3-acetylpyridine, harmaline and nicotinamide in Sprague-Dawley and Long-Evans rats: a critical review of 3-acetyl-pyridine neurotoxicity. Brain Res Rev 9: 21–42, 1985. - PubMed
-
- Bardin JM, Batini C, Billard JM, Buisseret-Delmas C, Conrath-Verrier M, Corvaja N. Cerebellar output regulation by the climbing and mossy fibers with and without the inferior olive. J Comp Neurol 213: 464–477, 1983. - PubMed
-
- Benedetti F, Montarolo PG, Rabacchi S. Inferior olive lesion induces long-lasting functional modification in the Purkinje cells. Exp Brain Res 55: 368–371, 1984. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

