Is realistic neuronal modeling realistic?
- PMID: 27535372
- PMCID: PMC5102320
- DOI: 10.1152/jn.00360.2016
Is realistic neuronal modeling realistic?
Abstract
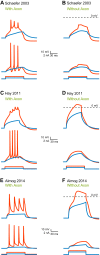
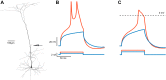
Scientific models are abstractions that aim to explain natural phenomena. A successful model shows how a complex phenomenon arises from relatively simple principles while preserving major physical or biological rules and predicting novel experiments. A model should not be a facsimile of reality; it is an aid for understanding it. Contrary to this basic premise, with the 21st century has come a surge in computational efforts to model biological processes in great detail. Here we discuss the oxymoronic, realistic modeling of single neurons. This rapidly advancing field is driven by the discovery that some neurons don't merely sum their inputs and fire if the sum exceeds some threshold. Thus researchers have asked what are the computational abilities of single neurons and attempted to give answers using realistic models. We briefly review the state of the art of compartmental modeling highlighting recent progress and intrinsic flaws. We then attempt to address two fundamental questions. Practically, can we realistically model single neurons? Philosophically, should we realistically model single neurons? We use layer 5 neocortical pyramidal neurons as a test case to examine these issues. We subject three publically available models of layer 5 pyramidal neurons to three simple computational challenges. Based on their performance and a partial survey of published models, we conclude that current compartmental models are ad hoc, unrealistic models functioning poorly once they are stretched beyond the specific problems for which they were designed. We then attempt to plot possible paths for generating realistic single neuron models.
Keywords: cable theory; channel kinetics; compartmental model; dendrites; ion channel.
Copyright © 2016 the American Physiological Society.
Figures



References
-
- Abeles M. Corticonics: Neural Circuits of the Cerebral Cortex. Cambridge, UK: Cambridge University Press, 1991, p. xiv, 280.
-
- Amitai Y, Friedman A, Connors BW, Gutnick MJ. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cereb Cortex 3: 26–38, 1993. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

