Fascin regulates nuclear actin during Drosophila oogenesis
- PMID: 27535426
- PMCID: PMC5042582
- DOI: 10.1091/mbc.E15-09-0634
Fascin regulates nuclear actin during Drosophila oogenesis
Abstract
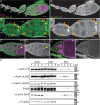
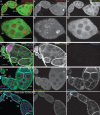
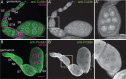
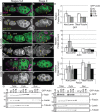
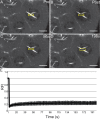
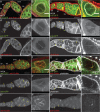
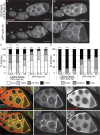
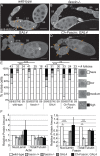
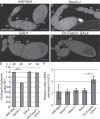
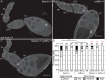
Drosophila oogenesis provides a developmental system with which to study nuclear actin. During Stages 5-9, nuclear actin levels are high in the oocyte and exhibit variation within the nurse cells. Cofilin and Profilin, which regulate the nuclear import and export of actin, also localize to the nuclei. Expression of GFP-tagged Actin results in nuclear actin rod formation. These findings indicate that nuclear actin must be tightly regulated during oogenesis. One factor mediating this regulation is Fascin. Overexpression of Fascin enhances nuclear GFP-Actin rod formation, and Fascin colocalizes with the rods. Loss of Fascin reduces, whereas overexpression of Fascin increases, the frequency of nurse cells with high levels of nuclear actin, but neither alters the overall nuclear level of actin within the ovary. These data suggest that Fascin regulates the ability of specific cells to accumulate nuclear actin. Evidence indicates that Fascin positively regulates nuclear actin through Cofilin. Loss of Fascin results in decreased nuclear Cofilin. In addition, Fascin and Cofilin genetically interact, as double heterozygotes exhibit a reduction in the number of nurse cells with high nuclear actin levels. These findings are likely applicable beyond Drosophila follicle development, as the localization and functions of Fascin and the mechanisms regulating nuclear actin are widely conserved.
© 2016 Kelpsch et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
References
-
- Abramoff M, Magalhaes P, Ram S. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42.
-
- Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem. 1995;270:17582–17587. - PubMed
-
- Asumda FZ, Chase PB. Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation. 2012;83:106–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

